Percutaneous ultrasound-guided cryoablation for early-stage primary breast cancer: a follow-up study in Japan
- PMID: 38678120
- PMCID: PMC11194206
- DOI: 10.1007/s12282-024-01584-4
Percutaneous ultrasound-guided cryoablation for early-stage primary breast cancer: a follow-up study in Japan
Abstract
Background: Ultrasound-guided percutaneous cryoablation (PCA) for early-stage breast cancer (ESBC) can be performed under local anesthesia in an outpatient clinic. This study continues a pilot stage to examine local control, safety, patient quality of life (QoL), satisfaction and cosmetic outcomes of cryoablation for ESBC.
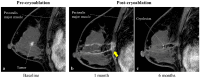
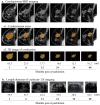
Methods: PCA was performed under local anesthesia for patients with primary ESBC, followed by radiation and endocrine therapies. Oncologic outcomes were examined by imaging (mammography, ultrasound, MRI) at baseline and 1, 6, 12, 24, 36, and 60 months post-cryoablation. EQ-VAS, EQ-5D-5L, subjective satisfaction and Moiré topography were used to measure health-related QoL outcomes.
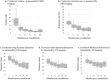
Results: Eighteen patients, mean aged 59.0 ± 9.0 years, mean tumor size 9.8 ± 2.3 mm, ER + , PR + (17/18), HER2-, Ki67 < 20% (15/18), underwent PCA and were followed for a mean of 44.3 months. No serious adverse events were reported, and no patients had local recurrence or distant metastasis in the 5-year follow-up. Cosmetic outcomes, satisfaction level, and QoL all improved post-cryoablation. Five-year average reduction rates of the cryolesion long, short, and depth diameters, on US, were 61.3%, 42.3%, and 22.8%, respectively, compared to the 86.2% volume reduction rate on MRI. The correlation coefficient between MRI and US measurement criteria was highest for the long diameter. During follow-up, calcification of the treated area was observed in 13/18 cases.
Conclusion: Cryoablation for ESBC is an effective and safe procedure with excellent cosmetic outcomes and improved QoL. This study contributes to the growing evidence supporting cryoablation as a potential standard treatment for ESBC, given compliance to pre-defined patient selection criteria.
Keywords: Breast cancer; Cryoablation; Cryotherapy; Nonsurgical ablation; Outpatient procedure.
© 2024. The Author(s).
Conflict of interest statement
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Ultrasound-guided cryoablation of early breast cancer: safety, technical efficacy, patients' satisfaction, and outcome prediction with MRI/CEM: a pilot case-control study.Eur Radiol Exp. 2024 Oct 22;8(1):120. doi: 10.1186/s41747-024-00515-4. Eur Radiol Exp. 2024. PMID: 39436590 Free PMC article.
-
A Pilot Study of Ultrasound-Guided Cryoablation of Invasive Ductal Carcinomas up to 15 mm With MRI Follow-Up and Subsequent Surgical Resection.AJR Am J Roentgenol. 2015 May;204(5):1100-8. doi: 10.2214/AJR.13.12325. AJR Am J Roentgenol. 2015. PMID: 25905948 Free PMC article.
-
Ultrasound guided cryoablation of fibroadenomas.Ultraschall Med. 2013 Feb;34(1):64-8. doi: 10.1055/s-0032-1325460. Epub 2012 Nov 9. Ultraschall Med. 2013. PMID: 23143883
-
Percutaneous cryoablation of hepatic tumors: long-term experience of a large U.S. series.Abdom Radiol (NY). 2016 Apr;41(4):767-80. doi: 10.1007/s00261-016-0687-x. Abdom Radiol (NY). 2016. PMID: 26960728 Review.
-
Percutaneous Image-Guided Cryoablation of Breast Cancer: A Systematic Review.J Vasc Interv Radiol. 2015 Nov;26(11):1652-7.e1. doi: 10.1016/j.jvir.2015.07.020. Epub 2015 Sep 3. J Vasc Interv Radiol. 2015. PMID: 26342882 Review.
Cited by
-
Cryoablation Without Excision for Early-Stage Breast Cancer: ICE3 Trial 5-Year Follow-Up on Ipsilateral Breast Tumor Recurrence.Ann Surg Oncol. 2024 Oct;31(11):7273-7283. doi: 10.1245/s10434-024-16181-0. Epub 2024 Sep 16. Ann Surg Oncol. 2024. PMID: 39283572 Free PMC article.
-
Local recurrence and residual tumor rates following cryoablation for small early-stage breast cancers: systemic review and meta-analysis.Breast Cancer. 2025 Jan;32(1):69-78. doi: 10.1007/s12282-024-01643-w. Epub 2024 Oct 19. Breast Cancer. 2025. PMID: 39425821 Review.
References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

