New Pyranone Derivatives and Sesquiterpenoid Isolated from the Endophytic Fungus Xylaria sp. Z184
- PMID: 38675548
- PMCID: PMC11051921
- DOI: 10.3390/molecules29081728
New Pyranone Derivatives and Sesquiterpenoid Isolated from the Endophytic Fungus Xylaria sp. Z184
Abstract
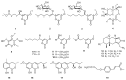
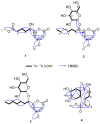
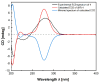
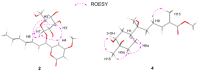
The fungus Xylaria sp. Z184, harvested from the leaves of Fallopia convolvulus (L.) Á. Löve, has been isolated for the first time. Chemical investigation on the methanol extract of the culture broth of the titles strain led to the discovery of three new pyranone derivatives, called fallopiaxylaresters A-C (1-3), and a new bisabolane-type sesquiterpenoid, named fallopiaxylarol A (4), along with the first complete set of spectroscopic data for the previously reported pestalotiopyrone M (5). Known pyranone derivatives (6-11), sesquiterpenoids (12-14), isocoumarin derivatives (15-17), and an aromatic allenic ether (18) were also co-isolated in this study. All new structures were elucidated by the interpretation of HRESIMS, 1D, 2D NMR spectroscopy, and quantum chemical computation approach. The in vitro antimicrobial, anti-inflammatory, and α-glucosidase-inhibitory activities of the selected compounds and the crude extract were evaluated. The extract was shown to inhibit nitric oxide (NO) production induced by lipopolysaccharide (LPS) in murine RAW264.7 macrophage cells, with an inhibition rate of 77.28 ± 0.82% at a concentration of 50 μg/mL. The compounds 5, 7, and 8 displayed weak antibacterial activity against Staphylococcus areus subsp. aureus at a concentration of 100 μM.
Keywords: Xylaria sp.; antimicrobial activity; pyranone derivative; sesquiterpenoid.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Caryophyllene sesquiterpenoids from the endophytic fungus, Pestalotiopsis sp.Fitoterapia. 2016 Mar;109:119-24. doi: 10.1016/j.fitote.2015.12.003. Epub 2015 Dec 11. Fitoterapia. 2016. PMID: 26687557
-
Four new sesquiterpenoids with anti-inflammatory activity from the stems of Jasminum officinale.Fitoterapia. 2019 Jun;135:22-26. doi: 10.1016/j.fitote.2019.03.029. Epub 2019 Apr 1. Fitoterapia. 2019. PMID: 30946945
-
Neural anti-inflammatory sesquiterpenoids from the endophytic fungus Trichoderma sp. Xy24.J Asian Nat Prod Res. 2017 Jul;19(7):651-658. doi: 10.1080/10286020.2016.1251908. Epub 2016 Nov 11. J Asian Nat Prod Res. 2017. PMID: 27835936
-
Sesquiterpenoids from Tussilago farfara inhibit LPS-induced nitric oxide production in macrophage RAW 264.7 cells.Arch Pharm Res. 2016 Jan;39(1):127-32. doi: 10.1007/s12272-015-0667-7. Epub 2015 Oct 17. Arch Pharm Res. 2016. PMID: 26474586
-
Chlomultiols A-L, sesquiterpenoids from Chloranthus multistachys and their anti-inflammatory activities.Phytochemistry. 2022 Jan;193:113001. doi: 10.1016/j.phytochem.2021.113001. Epub 2021 Nov 8. Phytochemistry. 2022. PMID: 34763221 Review.
References
-
- Yoiprommarat S., Unagul P., Suvannakad R., Klaysuban A., Suetrong S., Bunyapaiboonsri T. Eremophilane sesquiterpenes from the mangrove fungus BCC 60405. Phytochem. Lett. 2019;34:84–90. doi: 10.1016/j.phytol.2019.09.005. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

