Evaluation of a Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)-Based Microneutralization Assay for Assessing Clinical Human Cytomegalovirus-Neutralizing Antibody Activity
- PMID: 38674686
- PMCID: PMC11052257
- DOI: 10.3390/microorganisms12040742
Evaluation of a Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)-Based Microneutralization Assay for Assessing Clinical Human Cytomegalovirus-Neutralizing Antibody Activity
Abstract
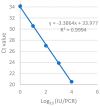
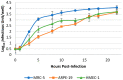
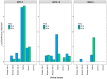
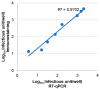
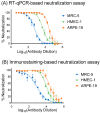
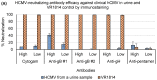
Development of a vaccine for human cytomegalovirus (hCMV) is critical because of the severe consequences of infection in congenitally infected newborns and immunocompromised patients. The assessment of hCMV-neutralizing antibody activity is crucial for vaccine development. This study evaluated a RT-qPCR assay targeting the immediate-early gene transcript of hCMV for determining microneutralizing antibody activity. The assay was evaluated for sensitivity, specificity, and precision using endotheliotropic clinical isolate VR1814 that infects fibroblasts, epithelial, and endothelial cells. The RT-qPCR-based neutralization assay was compared with an immunostaining-based neutralization assay using virions present in hCMV-positive urine, saliva, and breast-milk samples. Our results showed that hCMV replication was detectable at 20 h post-infection with a limit of detection of 1 infectious units (IU)/reaction. The RT-qPCR assay had a dynamic range of 1 to 1.0 × 104 IU/reaction, with coefficients of variation ranging from 0.94% to 15.08%. The RT-qPCR results were in high agreement with the immunostaining assay for hCMV-antibody neutralization assessment. Overall, the RT-qPCR neutralization assay is a reliable, rapid, efficient, and sensitive alternative method for evaluating hCMV-neutralizing activity in vitro.
Keywords: RT-qPCR; human cytomegalovirus; immunostaining assay; neutralization; viral mRNA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
RT-qPCR-based microneutralization assay for human cytomegalovirus using fibroblasts and epithelial cells.Vaccine. 2015 Dec 16;33(51):7254-7261. doi: 10.1016/j.vaccine.2015.10.110. Epub 2015 Nov 6. Vaccine. 2015. PMID: 26552003
-
Molecular diagnosis of Cytomegalovirus infection: clinical performance of the Aptima transcription-mediated amplification assay toward conventional qPCR chemistry on whole blood samples.J Clin Microbiol. 2024 Mar 13;62(3):e0090623. doi: 10.1128/jcm.00906-23. Epub 2024 Feb 13. J Clin Microbiol. 2024. PMID: 38349144 Free PMC article.
-
Neutralization of Diverse Human Cytomegalovirus Strains Conferred by Antibodies Targeting Viral gH/gL/pUL128-131 Pentameric Complex.J Virol. 2017 Mar 13;91(7):e02033-16. doi: 10.1128/JVI.02033-16. Print 2017 Apr 1. J Virol. 2017. PMID: 28077654 Free PMC article.
-
Preliminary Evaluation of Next-Generation Sequencing Performance Relative to qPCR and In Vitro Cell Culture Tests for Human Cytomegalovirus.PDA J Pharm Sci Technol. 2014 Nov-Dec;68(6):563-71. doi: 10.5731/pdajpst.2014.01013. PDA J Pharm Sci Technol. 2014. PMID: 25475630
-
Humoral immune response to human cytomegalovirus infection: diagnostic potential of immunoglobulin class and IgG subclass antibody response to human cytomegalovirus early and late antigens.Clin Investig. 1993 Apr;71(4):270-6. doi: 10.1007/BF00184725. Clin Investig. 1993. PMID: 8386033 Review.
References
-
- Louten J. Essential Human Virology. Academic Press; Cambridge, MA, USA: 2016. Herpesviruses; pp. 235–256.
Grants and funding
LinkOut - more resources
Full Text Sources

