Identification of Key Genes and Imbalanced SNAREs Assembly in the Comorbidity of Polycystic Ovary Syndrome and Depression
- PMID: 38674428
- PMCID: PMC11049873
- DOI: 10.3390/genes15040494
Identification of Key Genes and Imbalanced SNAREs Assembly in the Comorbidity of Polycystic Ovary Syndrome and Depression
Abstract
Background: Women with polycystic ovary syndrome (PCOS) have increased odds of concurrent depression, indicating that the relationship between PCOS and depression is more likely to be comorbid. However, the underlying mechanism remains unclear. Here, we aimed to use bioinformatic analysis to screen for the genetic elements shared between PCOS and depression.
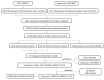
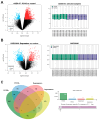
Methods: Differentially expressed genes (DEGs) were screened out through GEO2R using the PCOS and depression datasets in NCBI. Protein-protein interaction (PPI) network analysis and enrichment analysis were performed to identify the potential hub genes. After verification using other PCOS and depression datasets, the associations between key gene polymorphism and comorbidity were further studied using data from the UK biobank (UKB) database.
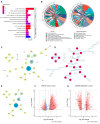
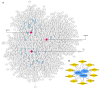
Results: In this study, three key genes, namely, SNAP23, VTI1A, and PRKAR1A, and their related SNARE interactions in the vesicular transport pathway were identified in the comorbidity of PCOS and depression. The rs112568544 at SNAP23, rs11077579 and rs4458066 at PRKAR1A, and rs10885349 at VTI1A might be the genetic basis of this comorbidity.
Conclusions: Our study suggests that the SNAP23, PRKAR1A, and VTI1A genes can directly or indirectly participate in the imbalanced assembly of SNAREs in the pathogenesis of the comorbidity of PCOS and depression. These findings may provide new strategies in diagnosis and therapy for this comorbidity.
Keywords: SNAREs; comorbidity; depression; polycystic ovary syndrome (PCOS); single nucleotide polymorphisms (SNPs).
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Identification of key pathways and genes in polycystic ovary syndrome via integrated bioinformatics analysis and prediction of small therapeutic molecules.Reprod Biol Endocrinol. 2021 Feb 23;19(1):31. doi: 10.1186/s12958-021-00706-3. Reprod Biol Endocrinol. 2021. PMID: 33622336 Free PMC article.
-
Bioinformatics-based analysis of potential therapeutic target genes for polycystic ovary syndrome.Cell Mol Biol (Noisy-le-grand). 2024 Apr 28;70(4):169-175. doi: 10.14715/cmb/2024.70.4.27. Cell Mol Biol (Noisy-le-grand). 2024. PMID: 38678611
-
Identification of Hub Genes and Biomarkers between Hyperandrogen and Normoandrogen Polycystic Ovary Syndrome by Bioinformatics Analysis.Comb Chem High Throughput Screen. 2023;26(1):126-134. doi: 10.2174/1386207325666220404101009. Comb Chem High Throughput Screen. 2023. PMID: 35379124
-
High prevalence of moderate and severe depressive and anxiety symptoms in polycystic ovary syndrome: a systematic review and meta-analysis.Hum Reprod. 2017 May 1;32(5):1075-1091. doi: 10.1093/humrep/dex044. Hum Reprod. 2017. PMID: 28333286 Review.
-
Polymorphisms of Cytokine Genes and Polycystic Ovary Syndrome: A Review.Metab Syndr Relat Disord. 2016 Dec;14(10):468-474. doi: 10.1089/met.2016.0101. Epub 2016 Nov 3. Metab Syndr Relat Disord. 2016. PMID: 27809669 Review.
References
-
- Bhandary P., Shetty P.K., Manjeera L., Patil P. Hormonal, genetic, epigenetic and environmental aspects of polycystic ovarian syndrome. Gene Rep. 2022;29:101698. doi: 10.1016/j.genrep.2022.101698. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

