DNA Damage and Parkinson's Disease
- PMID: 38673772
- PMCID: PMC11050701
- DOI: 10.3390/ijms25084187
DNA Damage and Parkinson's Disease
Abstract
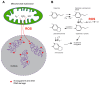
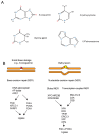
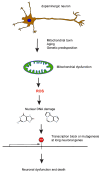
The etiology underlying most sporadic Parkinson's' disease (PD) cases is unknown. Environmental exposures have been suggested as putative causes of the disease. In cell models and in animal studies, certain chemicals can destroy dopaminergic neurons. However, the mechanisms of how these chemicals cause the death of neurons is not understood. Several of these agents are mitochondrial toxins that inhibit the mitochondrial complex I of the electron transport chain. Familial PD genes also encode proteins with important functions in mitochondria. Mitochondrial dysfunction of the respiratory chain, in combination with the presence of redox active dopamine molecules in these cells, will lead to the accumulation of reactive oxygen species (ROS) in dopaminergic neurons. Here, I propose a mechanism regarding how ROS may lead to cell killing with a specificity for neurons. One rarely considered hypothesis is that ROS produced by defective mitochondria will lead to the formation of oxidative DNA damage in nuclear DNA. Many genes that encode proteins with neuron-specific functions are extraordinary long, ranging in size from several hundred kilobases to well over a megabase. It is predictable that such long genes will contain large numbers of damaged DNA bases, for example in the form of 8-oxoguanine (8-oxoG), which is a major DNA damage type produced by ROS. These DNA lesions will slow down or stall the progression of RNA polymerase II, which is a term referred to as transcription stress. Furthermore, ROS-induced DNA damage may cause mutations, even in postmitotic cells such as neurons. I propose that the impaired transcription and mutagenesis of long, neuron-specific genes will lead to a loss of neuronal integrity, eventually leading to the death of these cells during a human lifetime.
Keywords: DNA damage; DNA repair; Parkinson’s disease; mitochondria; mutations; transcription.
Conflict of interest statement
The author declares no conflict of interest.
Figures

Similar articles
-
Damage to dopaminergic neurons by oxidative stress in Parkinson's disease (Review).Int J Mol Med. 2018 Apr;41(4):1817-1825. doi: 10.3892/ijmm.2018.3406. Epub 2018 Jan 19. Int J Mol Med. 2018. PMID: 29393357 Review.
-
Reprint of: revisiting oxidative stress and mitochondrial dysfunction in the pathogenesis of Parkinson disease-resemblance to the effect of amphetamine drugs of abuse.Free Radic Biol Med. 2013 Sep;62:186-201. doi: 10.1016/j.freeradbiomed.2013.05.042. Epub 2013 Jun 3. Free Radic Biol Med. 2013. PMID: 23743292 Review.
-
Mitochondria-mediated damage to dopaminergic neurons in Parkinson's disease (Review).Int J Mol Med. 2018 Feb;41(2):615-623. doi: 10.3892/ijmm.2017.3255. Epub 2017 Nov 16. Int J Mol Med. 2018. PMID: 29207041 Review.
-
Cholesterol contributes to dopamine-neuronal loss in MPTP mouse model of Parkinson's disease: Involvement of mitochondrial dysfunctions and oxidative stress.PLoS One. 2017 Feb 7;12(2):e0171285. doi: 10.1371/journal.pone.0171285. eCollection 2017. PLoS One. 2017. PMID: 28170429 Free PMC article.
-
Oxidative Stress and Dopaminergic Metabolism: A Major PD Pathogenic Mechanism and Basis of Potential Antioxidant Therapies.CNS Neurol Disord Drug Targets. 2024;23(7):852-864. doi: 10.2174/1871527322666230609141519. CNS Neurol Disord Drug Targets. 2024. PMID: 37303175 Review.
Cited by
-
Linking Environmental Genotoxins to Neurodegenerative Diseases Through Transcriptional Mutagenesis.Int J Mol Sci. 2024 Oct 24;25(21):11429. doi: 10.3390/ijms252111429. Int J Mol Sci. 2024. PMID: 39518982 Free PMC article. Review.
References
-
- Nalls M.A., Blauwendraat C., Vallerga C.L., Heilbron K., Bandres-Ciga S., Chang D., Tan M., Kia D.A., Noyce A.J., Xue A., et al. Identification of novel risk loci, causal insights, and heritable risk for Parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019;18:1091–1102. doi: 10.1016/S1474-4422(19)30320-5. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

