Sustainable Valorization of Coffee Silverskin: Extraction of Phenolic Compounds and Proteins for Enzymatic Production of Bioactive Peptides
- PMID: 38672902
- PMCID: PMC11048817
- DOI: 10.3390/foods13081230
Sustainable Valorization of Coffee Silverskin: Extraction of Phenolic Compounds and Proteins for Enzymatic Production of Bioactive Peptides
Abstract
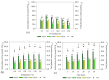
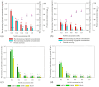
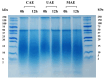
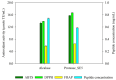
Coffee silverskin (CS), a by-product of the coffee roasting process, has high protein content (16.2-19.0%, w/w), making it a potential source for plant protein and bioactive peptide production. This study aims to develop innovative extraction methods for phenolic compounds and proteins from CS. The conditions for hydrothermal (HT) extraction of phenolic compounds from CS were optimized by varying CS loading (2.5-10%, w/v), temperature (110-130 °C), and time (5-30 min) using a one-factor-at-a-time (OFAT) approach. The highest TPC of 55.59 ± 0.12 µmole GAE/g CS was achieved at 5.0% (w/v) CS loading and autoclaving at 125 °C for 25 min. Following hydrothermal extraction, CS protein was extracted from HT-extracted solid fraction by microwave-assisted alkaline extraction (MAE) using 0.2 M NaOH at 90 W for 2 min, resulting in a protein recovery of 12.19 ± 0.39 mg/g CS. The CS protein was then subjected to enzymatic hydrolysis using protease from Bacillus halodurans SE5 (protease_SE5). Protease_SE5-derived CS protein hydrolysate had a peptide concentration of 0.73 ± 0.09 mg/mL, with ABTS, DPPH, and FRAP values of 15.71 ± 0.10, 16.63 ± 0.061, and 6.48 ± 0.01 µmole TE/mL, respectively. Peptide identification by LC-MS/MS revealed several promising biological activities without toxicity or allergenicity concerns. This study's integrated approach offers a sustainable and efficient method for extracting valuable compounds from CS, with potential applications in the food and pharmaceutical industries.
Keywords: bioactive compounds; coffee silverskin valorization; hydrothermal extraction; microwave-assisted alkaline extraction; phenolic compounds; protein hydrolysis; ultrasound-assisted alkaline extraction.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
Similar articles
-
Optimization and kinetics modeling of phenolics extraction from coffee silverskin in deep eutectic solvent using ultrasound-assisted extraction.Heliyon. 2023 Jul 4;9(7):e17942. doi: 10.1016/j.heliyon.2023.e17942. eCollection 2023 Jul. Heliyon. 2023. PMID: 37449125 Free PMC article.
-
Optimization of ultrasound-assisted extraction of bioactive compounds from coffee pulp using propylene glycol as a solvent and their antioxidant activities.Ultrason Sonochem. 2022 Sep;89:106127. doi: 10.1016/j.ultsonch.2022.106127. Epub 2022 Aug 18. Ultrason Sonochem. 2022. PMID: 36007328 Free PMC article.
-
Microwave-Assisted Extraction of Phenolic Compounds and Antioxidants for Cosmetic Applications Using Polyol-Based Technology.J Vis Exp. 2024 Aug 23;(210). doi: 10.3791/67033. J Vis Exp. 2024. PMID: 39248485
-
Utilization of Coffee Silverskin By-Product from Coffee Roasting Industry through Extraction Process for the Development of Antioxidant Skin Gel.J Cosmet Sci. 2019 Nov/Dec;70(6):313-325. J Cosmet Sci. 2019. PMID: 31829924
-
Valorization of coffee pulp as bioactive food ingredient by sustainable extraction methodologies.Curr Res Food Sci. 2023 Mar 1;6:100475. doi: 10.1016/j.crfs.2023.100475. eCollection 2023. Curr Res Food Sci. 2023. PMID: 36935849 Free PMC article. Review.
Cited by
-
Phenolic Compositions of Different Fractions from Coffee Silver Skin and Their Antioxidant Activities and Inhibition towards Carbohydrate-Digesting Enzymes.Foods. 2024 Sep 27;13(19):3083. doi: 10.3390/foods13193083. Foods. 2024. PMID: 39410118 Free PMC article.
References
-
- Nolasco A., Squillante J., Velotto S., D’Auria G., Ferranti P., Mamone G., Errico M.E., Avolio R., Castaldo R., Cirillo T., et al. Valorization of coffee industry wastes: Comprehensive physicochemical characterization of coffee silverskin and multipurpose recycling applications. J. Clean. Prod. 2022;370:133520. doi: 10.1016/j.jclepro.2022.133520. - DOI
-
- Narita Y., Inouye K. Review on utilization and composition of coffee silverskin. Food Res. Int. 2014;61:16–22. doi: 10.1016/j.foodres.2014.01.023. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

