Recent Findings on Therapeutic Cancer Vaccines: An Updated Review
- PMID: 38672519
- PMCID: PMC11048403
- DOI: 10.3390/biom14040503
Recent Findings on Therapeutic Cancer Vaccines: An Updated Review
Abstract
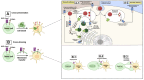
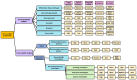
Cancer remains one of the global leading causes of death and various vaccines have been developed over the years against it, including cell-based, nucleic acid-based, and viral-based cancer vaccines. Although many vaccines have been effective in in vivo and clinical studies and some have been FDA-approved, there are major limitations to overcome: (1) developing one universal vaccine for a specific cancer is difficult, as tumors with different antigens are different for different individuals, (2) the tumor antigens may be similar to the body's own antigens, and (3) there is the possibility of cancer recurrence. Therefore, developing personalized cancer vaccines with the ability to distinguish between the tumor and the body's antigens is indispensable. This paper provides a comprehensive review of different types of cancer vaccines and highlights important factors necessary for developing efficient cancer vaccines. Moreover, the application of other technologies in cancer therapy is discussed. Finally, several insights and conclusions are presented, such as the possibility of using cold plasma and cancer stem cells in developing future cancer vaccines, to tackle the major limitations in the cancer vaccine developmental process.
Keywords: cancer vaccines; cold plasma; immunotherapy; stem cells.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
[Identification of cancer-stem cell antigens and development of CTL-mediated cancer immunotherapy].Nihon Rinsho Meneki Gakkai Kaishi. 2017;40(1):40-47. doi: 10.2177/jsci.40.40. Nihon Rinsho Meneki Gakkai Kaishi. 2017. PMID: 28539553 Review. Japanese.
-
Neoantigen: A New Breakthrough in Tumor Immunotherapy.Front Immunol. 2021 Apr 16;12:672356. doi: 10.3389/fimmu.2021.672356. eCollection 2021. Front Immunol. 2021. PMID: 33936118 Free PMC article. Review.
-
A New Approach for Cancer Immunotherapy Based on the Cancer Stem Cell Antigens Properties.Curr Mol Med. 2019;19(1):2-11. doi: 10.2174/1566524019666190204114721. Curr Mol Med. 2019. PMID: 30714514 Review.
-
A novel era of cancer/testis antigen in cancer immunotherapy.Int Immunopharmacol. 2021 Sep;98:107889. doi: 10.1016/j.intimp.2021.107889. Epub 2021 Jun 24. Int Immunopharmacol. 2021. PMID: 34174699 Review.
-
Therapeutic cancer vaccines: From initial findings to prospects.Immunol Lett. 2018 Apr;196:11-21. doi: 10.1016/j.imlet.2018.01.011. Epub 2018 Feb 4. Immunol Lett. 2018. PMID: 29407608 Review.
Cited by
-
Short Peptides as Powerful Arsenal for Smart Fighting Cancer.Cancers (Basel). 2024 Sep 24;16(19):3254. doi: 10.3390/cancers16193254. Cancers (Basel). 2024. PMID: 39409876 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

