Co-Treatment with Phlorotannin and Extracellular Vesicles from Ecklonia cava Inhibits UV-Induced Melanogenesis
- PMID: 38671856
- PMCID: PMC11047619
- DOI: 10.3390/antiox13040408
Co-Treatment with Phlorotannin and Extracellular Vesicles from Ecklonia cava Inhibits UV-Induced Melanogenesis
Abstract
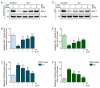
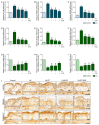
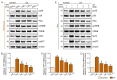
Hyperpigmentation due to ultraviolet (UV)-induced melanogenesis causes various esthetic problems. Phlorotannin (PT) and extracellular vesicles (EVs) derived from various plants suppress melanogenesis pathways. We used UV-exposed keratinocytes and animal skin to determine if co-treatment with PT and EVs from Ecklonia cava (EVE) could inhibit melanogenesis by reducing UV-induced oxidative stress and the expression of the thioredoxin-interacting protein (TXNIP)/nucleotide-binding oligomerization domain-like receptor family pyrin domain containing the 3 (NLRP3)/interleukin-18 (IL-18) pathway, which are upstream signals of the microphthalmia-associated transcription factor. UV exposure increased oxidative stress in keratinocytes and animal skin, as evaluated by 8-OHdG expression, and this effect was reduced by co-treatment with PT and EVE. UV also increased binding between NLRP3 and TXNIP, which increased NLRP3 inflammasome activation and IL-18 secretion, and this effect was reduced by co-treatment with PT and EVE in keratinocytes and animal skin. In melanocytes, conditioned media (CM) from UV-exposed keratinocytes increased the expression of melanogenesis-related pathways; however, these effects were reduced with CM from UV-exposed keratinocytes treated with PT and EVE. Similarly, PT and EVE treatment reduced melanogenesis-related signals, melanin content, and increased basement membrane (BM) components in UV-exposed animal skin. Thus, co-treatment with PT and EVE reduced melanogenesis and restored the BM structure by reducing oxidative stress and TXNIP/NLRP3/IL-18 pathway expression.
Keywords: TXNIP/NLRP3/IL-18 pathway; extracellular vesicles from Ecklonia cava; melanogenesis; phlorotannin; ultraviolet.
Conflict of interest statement
Kyunghee Byun has received research grants from LIBON Inc. and Kyung-A Byun is employed by LIBON Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Figures




Similar articles
-
Fermented Fish Collagen Attenuates Melanogenesis via Decreasing UV-Induced Oxidative Stress.Mar Drugs. 2024 Sep 15;22(9):421. doi: 10.3390/md22090421. Mar Drugs. 2024. PMID: 39330302 Free PMC article.
-
Extracellular Vesicles from Ecklonia cava and Phlorotannin Promote Rejuvenation in Aged Skin.Mar Drugs. 2024 May 15;22(5):223. doi: 10.3390/md22050223. Mar Drugs. 2024. PMID: 38786614 Free PMC article.
-
Radiofrequency Irradiation Attenuated UVB-Induced Skin Pigmentation by Modulating ATP Release and CD39 Expression.Int J Mol Sci. 2023 Mar 14;24(6):5506. doi: 10.3390/ijms24065506. Int J Mol Sci. 2023. PMID: 36982581 Free PMC article.
-
Recent development of signaling pathways inhibitors of melanogenesis.Cell Signal. 2017 Dec;40:99-115. doi: 10.1016/j.cellsig.2017.09.004. Epub 2017 Sep 12. Cell Signal. 2017. PMID: 28911859 Review.
-
Genetic and Epigenetic Regulation of the Innate Immune Response to Gout.Immunol Invest. 2023 Apr;52(3):364-397. doi: 10.1080/08820139.2023.2168554. Epub 2023 Feb 6. Immunol Invest. 2023. PMID: 36745138 Review.
Cited by
-
Radiofrequency Treatment Attenuates Age-Related Changes in Dermal-Epidermal Junctions of Animal Skin.Int J Mol Sci. 2024 May 9;25(10):5178. doi: 10.3390/ijms25105178. Int J Mol Sci. 2024. PMID: 38791217 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

