Iguratimod prevents renal fibrosis in unilateral ureteral obstruction model mice by suppressing M2 macrophage infiltration and macrophage-myofibroblast transition
- PMID: 38666363
- PMCID: PMC11057400
- DOI: 10.1080/0886022X.2024.2327498
Iguratimod prevents renal fibrosis in unilateral ureteral obstruction model mice by suppressing M2 macrophage infiltration and macrophage-myofibroblast transition
Abstract
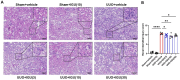
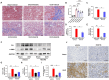
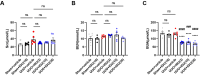
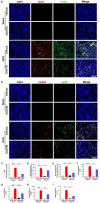
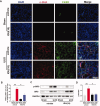
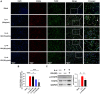
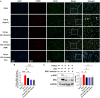
Iguratimod is a novel synthetic, small-molecule immunosuppressive agent used to treat rheumatoid arthritis. Through ongoing exploration of its role and mechanisms of action, iguratimod has been observed to have antifibrotic effects in the lung and skin; however, its effect on renal fibrosis remains unknown. This study aimed to investigate whether iguratimod could affect renal fibrosis progression. Three different concentrations of iguratimod (30 mg/kg/day, 10 mg/kg/day, and 3 mg/kg/day) were used to intervene in unilateral ureteral obstruction (UUO) model mice. Iguratimod at 10 mg/kg/day was observed to be effective in slowing UUO-mediated renal fibrosis. In addition, stimulating bone marrow-derived macrophages with IL-4 and/or iguratimod, or with TGF-β and iguratimod or SRC inhibitors in vitro, suggested that iguratimod mitigates the progression of renal fibrosis in UUO mice, at least in part, by inhibiting the IL-4/STAT6 signaling pathway to attenuate renal M2 macrophage infiltration, as well as by impeding SRC activation to reduce macrophage-myofibroblast transition. These findings reveal the potential of iguratimod as a treatment for renal disease.
Keywords: Iguratimod; macrophage infiltration; macrophage–myofibroblast transition; renal fibrosis; unilateral ureteral obstruction.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
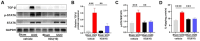
Similar articles
-
Pharmacological Inhibition of STAT6 Ameliorates Myeloid Fibroblast Activation and Alternative Macrophage Polarization in Renal Fibrosis.Front Immunol. 2021 Aug 26;12:735014. doi: 10.3389/fimmu.2021.735014. eCollection 2021. Front Immunol. 2021. PMID: 34512669 Free PMC article.
-
Delayed treatment with erythropoietin attenuates renal fibrosis in mouse model of unilateral ureteral obstruction.Int J Urol. 2024 Jun;31(6):685-692. doi: 10.1111/iju.15427. Epub 2024 Feb 17. Int J Urol. 2024. PMID: 38366861
-
Fate alteration of bone marrow-derived macrophages ameliorates kidney fibrosis in murine model of unilateral ureteral obstruction.Nephrol Dial Transplant. 2019 Oct 1;34(10):1657-1668. doi: 10.1093/ndt/gfy381. Nephrol Dial Transplant. 2019. PMID: 30590718
-
Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy.Kidney Int. 2009 Jun;75(11):1145-1152. doi: 10.1038/ki.2009.86. Epub 2009 Apr 1. Kidney Int. 2009. PMID: 19340094 Review.
-
The role of macrophages in fibrosis of chronic kidney disease.Biomed Pharmacother. 2024 Aug;177:117079. doi: 10.1016/j.biopha.2024.117079. Epub 2024 Jul 4. Biomed Pharmacother. 2024. PMID: 38968801 Review.
Cited by
-
Advances in macrophage-myofibroblast transformation in fibrotic diseases.Front Immunol. 2024 Oct 9;15:1461919. doi: 10.3389/fimmu.2024.1461919. eCollection 2024. Front Immunol. 2024. PMID: 39445007 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
