Adipocyte-released adipomes in Chagas cardiomyopathy: Impact on cardiac metabolic and immune regulation
- PMID: 38660407
- PMCID: PMC11039351
- DOI: 10.1016/j.isci.2024.109672
Adipocyte-released adipomes in Chagas cardiomyopathy: Impact on cardiac metabolic and immune regulation
Abstract
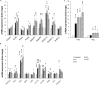
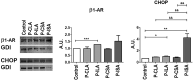
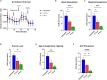
Chronic Trypanosoma cruzi infection leads to Chagas cardiomyopathy (CCM), with varying manifestations such as inflammatory hypertrophic cardiomyopathy, arrhythmias, and dilated cardiomyopathy. The factors responsible for the increasing risk of progression to CCM are not fully understood. Previous studies link adipocyte loss to CCM progression, but the mechanism triggering CCM pathogenesis remains unexplored. Our study uncovers that T. cruzi infection triggers adipocyte apoptosis, leading to the release of extracellular vesicles named "adipomes". We developed an innovative method to isolate intact adipomes from infected mice's adipose tissue and plasma, showing they carry unique lipid cargoes. Large and Small adipomes, particularly plasma-derived infection-associated L-adipomes (P-ILA), regulate immunometabolic signaling and induce cardiomyopathy. P-ILA treatment induces hypertrophic cardiomyopathy in wild-type mice and worsens cardiomyopathy severity in post-acute-infected mice by regulating adipogenic/lipogenic and mitochondrial functions. These findings highlight adipomes' pivotal role in promoting inflammation and impairing myocardial function during cardiac remodeling in CD.
Keywords: Biology experimental methods; Cell biology; Disease; Microbiology parasite.
© 2024 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review.
-
Using Experience Sampling Methodology to Capture Disclosure Opportunities for Autistic Adults.Autism Adulthood. 2023 Dec 1;5(4):389-400. doi: 10.1089/aut.2022.0090. Epub 2023 Dec 12. Autism Adulthood. 2023. PMID: 38116059 Free PMC article.
-
Dynamic Field Theory of Executive Function: Identifying Early Neurocognitive Markers.Monogr Soc Res Child Dev. 2024 Dec;89(3):7-109. doi: 10.1111/mono.12478. Monogr Soc Res Child Dev. 2024. PMID: 39628288 Free PMC article.
-
Qualitative evidence synthesis informing our understanding of people's perceptions and experiences of targeted digital communication.Cochrane Database Syst Rev. 2019 Oct 23;10(10):ED000141. doi: 10.1002/14651858.ED000141. Cochrane Database Syst Rev. 2019. PMID: 31643081 Free PMC article.
-
The effectiveness of abstinence-based and harm reduction-based interventions in reducing problematic substance use in adults who are experiencing homelessness in high income countries: A systematic review and meta-analysis: A systematic review.Campbell Syst Rev. 2024 Apr 21;20(2):e1396. doi: 10.1002/cl2.1396. eCollection 2024 Jun. Campbell Syst Rev. 2024. PMID: 38645303 Free PMC article. Review.
References
-
- Chadalawada S., Sillau S., Archuleta S., Mundo W., Bandali M., Parra-Henao G., Rodriguez-Morales A.J., Villamil-Gomez W.E., Suárez J.A., Shapiro L., et al. Risk of Chronic Cardiomyopathy Among Patients With the Acute Phase or Indeterminate Form of Chagas Disease: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.15072. - DOI - PMC - PubMed
-
- Nunes M.C.P., Beaton A., Acquatella H., Bern C., Bolger A.F., Echeverría L.E., Dutra W.O., Gascon J., Morillo C.A., Oliveira-Filho J., et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association. Circulation. 2018;138:e169–e209. doi: 10.1161/CIR.0000000000000599. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

