This is a preprint.
Transdifferentiation is uncoupled from progenitor pool expansion during hair cell regeneration in the zebrafish inner ear
- PMID: 38645220
- PMCID: PMC11030336
- DOI: 10.1101/2024.04.09.588777
Transdifferentiation is uncoupled from progenitor pool expansion during hair cell regeneration in the zebrafish inner ear
Update in
-
Transdifferentiation is temporally uncoupled from progenitor pool expansion during hair cell regeneration in the zebrafish inner ear.Development. 2024 Aug 1;151(15):dev202944. doi: 10.1242/dev.202944. Epub 2024 Aug 13. Development. 2024. PMID: 39045613 Free PMC article.
Abstract
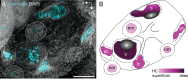
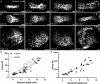
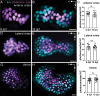
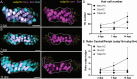
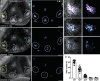
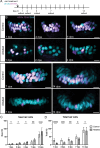
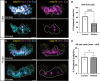
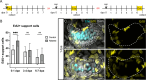
Death of mechanosensory hair cells in the inner ear is a common cause of auditory and vestibular impairment in mammals, which have a limited ability to regrow these cells after damage. In contrast, non-mammalian vertebrates including zebrafish can robustly regenerate hair cells following severe organ damage. The zebrafish inner ear provides an understudied model system for understanding hair cell regeneration in organs that are highly conserved with their mammalian counterparts. Here we quantitatively examine hair cell addition during growth and regeneration of the larval zebrafish inner ear. We used a genetically encoded ablation method to induce hair cell death and observed gradual regeneration with correct spatial patterning over two weeks following ablation. Supporting cells, which surround and are a source of new hair cells, divide in response to hair cell ablation, expanding the possible progenitor pool. In parallel, nascent hair cells arise from direct transdifferentiation of progenitor pool cells uncoupled from progenitor division. These findings reveal a previously unrecognized mechanism of hair cell regeneration with implications for how hair cells may be encouraged to regenerate in the mammalian ear.
Keywords: hair cell; inner ear; regeneration; transdifferentiation; zebrafish.
Conflict of interest statement
COMPETING INTERESTS No competing interests declared.
Figures
Similar articles
-
Transdifferentiation is temporally uncoupled from progenitor pool expansion during hair cell regeneration in the zebrafish inner ear.Development. 2024 Aug 1;151(15):dev202944. doi: 10.1242/dev.202944. Epub 2024 Aug 13. Development. 2024. PMID: 39045613 Free PMC article.
-
Single-cell transcriptomic profiling of the zebrafish inner ear reveals molecularly distinct hair cell and supporting cell subtypes.Elife. 2023 Jan 4;12:e82978. doi: 10.7554/eLife.82978. Elife. 2023. PMID: 36598134 Free PMC article.
-
Retinoic Acid Signaling Mediates Hair Cell Regeneration by Repressing p27kip and sox2 in Supporting Cells.J Neurosci. 2015 Nov 25;35(47):15752-66. doi: 10.1523/JNEUROSCI.1099-15.2015. J Neurosci. 2015. PMID: 26609166 Free PMC article.
-
Sensory hair cell regeneration in the zebrafish lateral line.Dev Dyn. 2014 Oct;243(10):1187-202. doi: 10.1002/dvdy.24167. Epub 2014 Aug 14. Dev Dyn. 2014. PMID: 25045019 Free PMC article. Review.
-
Hair cell regeneration in the avian auditory epithelium.Int J Dev Biol. 2007;51(6-7):633-47. doi: 10.1387/ijdb.072408js. Int J Dev Biol. 2007. PMID: 17891722 Review.
References
-
- Avallone B., Fascio U., Balsamo G. and Marmo F. (2008). Gentamicin ototoxicity in the saccule of the lizard Podarcis Sicula induces hair cell recovery and regeneration. Hear. Res. 235, 15–22. - PubMed
-
- Baird R. A., Steyger P. S. and Schuff N. R. (1996). Mitotic and nonmitotic hair cell regeneration in the bullfrog vestibular otolith organs. Ann. N. Y. Acad. Sci. 781, 59–70. - PubMed
-
- Bang P. I., Sewell W. F. and Malicki J. J. (2001). Morphology and cell type heterogeneities of the inner ear epithelia in adult and juvenile zebrafish (Danio rerio). J. Comp. Neurol. 438, 173–190. - PubMed
-
- Beck J. C., Gilland E., Tank D. W. and Baker R. (2004). Quantifying the ontogeny of optokinetic and vestibuloocular behaviors in zebrafish, medaka, and goldfish. J. Neurophysiol. 92, 3546–3561. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
