The protective effects of methylene blue on astrocytic swelling after cerebral ischemia-reperfusion injuries are mediated by Aquaporin-4 and metabotropic glutamate receptor 5 activation
- PMID: 38644842
- PMCID: PMC11031768
- DOI: 10.1016/j.heliyon.2024.e29483
The protective effects of methylene blue on astrocytic swelling after cerebral ischemia-reperfusion injuries are mediated by Aquaporin-4 and metabotropic glutamate receptor 5 activation
Abstract
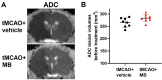
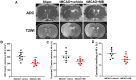
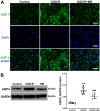
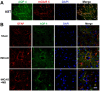
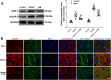
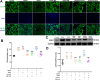
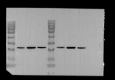
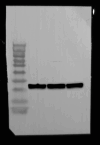
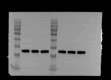
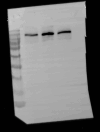
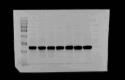
Methylene blue (MB) was found to exert neuroprotective effect on different brain diseases, such as ischemic stroke. This study assessed the MB effects on ischemia induced brain edema and its role in the inhibition of aquaporin 4 (AQP4) and metabotropic glutamate receptor 5 (mGluR5) expression. Rats were exposed 1 h transient middle cerebral artery occlusion (tMCAO), and MB was injected intravenously following reperfusion (3 mg/kg). Magnetic resonance imaging (MRI) and 2,3,5-triphenyltetrazolium chloride (TTC) staining was performed 48 h after the onset of tMCAO to evaluate the brain infarction and edema. Brain tissues injuries as well as the glial fibrillary acidic protein (GFAP), AQP4 and mGluR5 expressions were detected. Oxygen and glucose deprivation/reoxygenation (OGD/R) was performed on primary astrocytes (ASTs) to induce cell swelling. MB was administered at the beginning of reoxygenation, and the perimeter of ASTs was measured by GFAP immunofluorescent staining. 3,5-dihydroxyphenylglycine (DHPG) and fenobam were given at 24 h before OGD to examine their effects on MB functions on AST swelling and AQP4 expression. MB remarkably decreased the volumes of T2WI and ADC lesions, as well as the cerebral swelling. Consistently, MB treatment significantly decreased GFAP, mGluR5 and AQP4 expression at 48 h after stroke. In the cultivated primary ASTs, OGD/R and DHPG significantly increased ASTs volume as well as AQP4 expression, which was reversed by MB and fenobam treatment. The obtained results highlight that MB decreases the post-ischemic brain swelling by regulating the activation of AQP4 and mGluR5, suggesting potential applications of MB on clinical ischemic stroke treatment.
Keywords: AQP 4; Ischemic stroke; Methylene blue; brain edema; mGluR5.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Methylene blue ameliorates brain edema in rats with experimental ischemic stroke via inhibiting aquaporin 4 expression.Acta Pharmacol Sin. 2021 Mar;42(3):382-392. doi: 10.1038/s41401-020-0468-5. Epub 2020 Jul 14. Acta Pharmacol Sin. 2021. PMID: 32665706 Free PMC article.
-
Methylene Blue Ameliorates Ischemia/Reperfusion-Induced Cerebral Edema: An MRI and Transmission Electron Microscope Study.Acta Neurochir Suppl. 2016;121:227-36. doi: 10.1007/978-3-319-18497-5_41. Acta Neurochir Suppl. 2016. PMID: 26463954
-
Aquaporin 4-Mediated Glutamate-Induced Astrocyte Swelling Is Partially Mediated through Metabotropic Glutamate Receptor 5 Activation.Front Cell Neurosci. 2017 Apr 28;11:116. doi: 10.3389/fncel.2017.00116. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28503134 Free PMC article.
-
Remote ischemic post-conditioning improves neurological function by AQP4 down-regulation in astrocytes.Behav Brain Res. 2015 Aug 1;289:1-8. doi: 10.1016/j.bbr.2015.04.024. Epub 2015 Apr 21. Behav Brain Res. 2015. PMID: 25907740 Review.
-
Aquaporins in spinal cord injury: the janus face of aquaporin 4.Neuroscience. 2010 Jul 28;168(4):1019-35. doi: 10.1016/j.neuroscience.2010.01.037. Epub 2010 Jan 28. Neuroscience. 2010. PMID: 20109536 Free PMC article. Review.
References
-
- Mamtilahun M., Tang G., Zhang Z., Wang Y., Tang Y., Yang G.-Y. Targeting water in the brain: role of aquaporin-4 in ischemic brain edema. Curr. Drug Targets. 2019;20:748–755. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

