Trametinib sensitizes KRAS-mutant lung adenocarcinoma tumors to PD-1/PD-L1 axis blockade via Id1 downregulation
- PMID: 38643157
- PMCID: PMC11031964
- DOI: 10.1186/s12943-024-01991-3
Trametinib sensitizes KRAS-mutant lung adenocarcinoma tumors to PD-1/PD-L1 axis blockade via Id1 downregulation
Abstract
Background: The identification of novel therapeutic strategies to overcome resistance to the MEK inhibitor trametinib in mutant KRAS lung adenocarcinoma (LUAD) is a challenge. This study analyzes the effects of trametinib on Id1 protein, a key factor involved in the KRAS oncogenic pathway, and investigates the role of Id1 in the acquired resistance to trametinib as well as the synergistic anticancer effect of trametinib combined with immunotherapy in KRAS-mutant LUAD.
Methods: We evaluated the effects of trametinib on KRAS-mutant LUAD by Western blot, RNA-seq and different syngeneic mouse models. Genetic modulation of Id1 expression was performed in KRAS-mutant LUAD cells by lentiviral or retroviral transductions of specific vectors. Cell viability was assessed by cell proliferation and colony formation assays. PD-L1 expression and apoptosis were measured by flow cytometry. The anti-tumor efficacy of the combined treatment with trametinib and PD-1 blockade was investigated in KRAS-mutant LUAD mouse models, and the effects on the tumor immune infiltrate were analyzed by flow cytometry and immunohistochemistry.
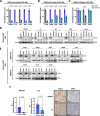
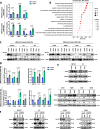
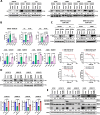
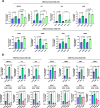
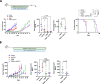
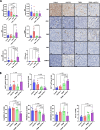
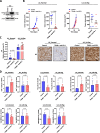
Results: We found that trametinib activates the proteasome-ubiquitin system to downregulate Id1 in KRAS-mutant LUAD tumors. Moreover, we found that Id1 plays a major role in the acquired resistance to trametinib treatment in KRAS-mutant LUAD cells. Using two preclinical syngeneic KRAS-mutant LUAD mouse models, we found that trametinib synergizes with PD-1/PD-L1 blockade to hamper lung cancer progression and increase survival. This anti-tumor activity depended on trametinib-mediated Id1 reduction and was associated with a less immunosuppressive tumor microenvironment and increased PD-L1 expression on tumor cells.
Conclusions: Our data demonstrate that Id1 expression is involved in the resistance to trametinib and in the synergistic effect of trametinib with anti-PD-1 therapy in KRAS-mutant LUAD tumors. These findings suggest a potential therapeutic approach for immunotherapy-refractory KRAS-mutant lung cancers.
Keywords: KRAS-mutant lung adenocarcinoma; Id1; PD-1 inhibition; PD-L1; Proteasome; Trametinib.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
The Combination of MEK Inhibitor With Immunomodulatory Antibodies Targeting Programmed Death 1 and Programmed Death Ligand 1 Results in Prolonged Survival in Kras/p53-Driven Lung Cancer.J Thorac Oncol. 2019 Jun;14(6):1046-1060. doi: 10.1016/j.jtho.2019.02.004. Epub 2019 Feb 13. J Thorac Oncol. 2019. PMID: 30771521 Free PMC article.
-
Inhibitor of Differentiation-1 Sustains Mutant KRAS-Driven Progression, Maintenance, and Metastasis of Lung Adenocarcinoma via Regulation of a FOSL1 Network.Cancer Res. 2019 Feb 1;79(3):625-638. doi: 10.1158/0008-5472.CAN-18-1479. Epub 2018 Dec 18. Cancer Res. 2019. PMID: 30563891
-
Trametinib downregulates survivin expression in RB1-positive KRAS-mutant lung adenocarcinoma cells.Biochem Biophys Res Commun. 2018 Jun 18;501(1):253-258. doi: 10.1016/j.bbrc.2018.04.230. Epub 2018 May 5. Biochem Biophys Res Commun. 2018. PMID: 29727601
-
Therapeutic potential of trametinib to inhibit the mutagenesis by inactivating the protein kinase pathway in non-small cell lung cancer.Expert Rev Anticancer Ther. 2019 Jan;19(1):11-17. doi: 10.1080/14737140.2019.1554440. Epub 2018 Dec 4. Expert Rev Anticancer Ther. 2019. PMID: 30513023 Review.
-
Intersecting evidence: Bibliometric analysis and clinical trials illuminate immunotherapy in KRAS-mutation cancer: A review.Medicine (Baltimore). 2024 Sep 6;103(36):e39334. doi: 10.1097/MD.0000000000039334. Medicine (Baltimore). 2024. PMID: 39252322 Review.
References
MeSH terms
Substances
Grants and funding
- PI20/00419/ISCIII-Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional
- PI19/00678/ISCIII-Fondo de Investigación Sanitaria-Fondo Europeo de Desarrollo Regional
- IDEAS211016AJON/Fundación Científica Asociación Española Contra el Cáncer
- 51-2021/Subvenciones para la promoción de proyectos de investigación por el Departamento de Salud 2021 Gobierno de Navarra
- 53/2021/Subvenciones para la promoción de proyectos de investigación por el Departamento de Salud 2021 Gobierno de Navarra
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

