Society for Immunotherapy of Cancer (SITC) recommendations on intratumoral immunotherapy clinical trials (IICT): from premalignant to metastatic disease
- PMID: 38641350
- PMCID: PMC11029323
- DOI: 10.1136/jitc-2023-008378
Society for Immunotherapy of Cancer (SITC) recommendations on intratumoral immunotherapy clinical trials (IICT): from premalignant to metastatic disease
Abstract
Background: Intratumorally delivered immunotherapies have the potential to favorably alter the local tumor microenvironment and may stimulate systemic host immunity, offering an alternative or adjunct to other local and systemic treatments. Despite their potential, these therapies have had limited success in late-phase trials for advanced cancer resulting in few formal approvals. The Society for Immunotherapy of Cancer (SITC) convened a panel of experts to determine how to design clinical trials with the greatest chance of demonstrating the benefits of intratumoral immunotherapy for patients with cancers across all stages of pathogenesis.
Methods: An Intratumoral Immunotherapy Clinical Trials Expert Panel composed of international key stakeholders from academia and industry was assembled. A multiple choice/free response survey was distributed to the panel, and the results of this survey were discussed during a half-day consensus meeting. Key discussion points are summarized in the following manuscript.
Results: The panel determined unique clinical trial designs tailored to different stages of cancer development-from premalignant to unresectable/metastatic-that can maximize the chance of capturing the effect of intratumoral immunotherapies. Design elements discussed included study type, patient stratification and exclusion criteria, indications of randomization, study arm determination, endpoints, biological sample collection, and response assessment with biomarkers and imaging. Populations to prioritize for the study of intratumoral immunotherapy, including stage, type of cancer and line of treatment, were also discussed along with common barriers to the development of these local treatments.
Conclusions: The SITC Intratumoral Immunotherapy Clinical Trials Expert Panel has identified key considerations for the design and implementation of studies that have the greatest potential to capture the effect of intratumorally delivered immunotherapies. With more effective and standardized trial designs, the potential of intratumoral immunotherapy can be realized and lead to regulatory approvals that will extend the benefit of these local treatments to the patients who need them the most.
Keywords: Clinical Trials as Topic; Guidelines as Topic; Immunotherapy; Melanoma.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: JJL: Researcher: AbbVie, Astellas, Astrazeneca, Bristol-Myers Squibb, Corvus, Day 1, EMD Serono, Fstar, Genmab, Ikena, Immatics, Incyte, Kadmon, KAHR, Macrogenics, Merck, Moderna, Nektar, Next Cure, Numab, Palleon, Pfizer, Replimmune, Rubius, Servier, Scholar Rock, Synlogic, Takeda, Trishula, Tizona, XencorConsultant/Advisor/Speaker: Abbvie, Agenus, Alnylam, Atomwise, Bayer, Bristol-Myers Squibb, Castle, Checkmate, Codiak, Crown, Cugene, Curadev, Day One, Eisai, EMD Serono, Endeavor, Flame, G1 Therapeutics, Genentech, Gilead, Glenmark, HotSpot, Kadmon, KSQ, Janssen, Ikena, Inzen, Immatics, Immunocore, Incyte, Instil, IO Biotech, Macrogenics, Merck, Mersana, Nektar, Novartis, Partner, Pfizer, Pioneering Medicines, PsiOxus, Regeneron, Replimmune, Ribon, Roivant, Servier, STINGthera, Synlogic, Synthekine, 7 Hills, Affivant, Bright Peak, Exo, Fstar, Inzen, RefleXion, Xilio (stock) Actym, Alphamab Oncology, Arch Oncology, Duke Street Bio, Kanaph, Mavu, NeoTx, Onc.AI, OncoNano, physIQ, Pyxis, Saros, STipe, Tempest, Abbvie, Agenus, Amgen, Immutep, Evaxion. DD: Grants/Research Support (institutional): Arcus, Immunocore, Merck, Regeneron Pharmaceuticals, Tesaro/GSK. Consultant: ACM Bio, Ascendis, Castle, Clinical Care Options (CCO), Gerson Lehrman Group (GLG), Immunitas, Medical Learning Group (MLG), Replimmune, Trisalus, Xilio Therapeutics. CE Speakers’ Bureau: Castle Biosciences. Stockholder: None. Intellectual Property: US Patent 63/124,231, "Compositions and Methods for Treating Cancer", December 11, 2020 US Patent 63/208,719, "Compositions and Methods For Responsiveness to Immune Checkpoint Inhibitors (ICI), Increasing Effectiveness of ICI and Treating Cancer", June 9, 2021. AM: Owner: HiFiBio, Deka Bio, Hotspot Therapeutics, Shattuck Labs, Researcher: Astra Zeneca, BMS, Sanofi, Consultant/Advisor/Speaker: Gritstone, Innate Pharma, Neogene, Deka Bio, Hotspot therapeutics, J&J, Medicxi, Depth Charge, BiolineRx, Clover Pharma, Grey Wolf, Lytix, RedX, HiFiBio, ImCheck, Applied Materials, Takeda, Shattuck Labs, Marengo therapeutics, Pierre Fabre, Third Rock Ventures, SotioPublicly Traded Stocks: Centessa. RHA–Employee: Seven & Eight Biopharmaceuticals. Ownership Interest Less Than 5 Percent: Seven & Eight Biopharmaceuticals. NB: Consulting Fees: Novartis, Apricity, Rome Therapeutics, BreakBio, Carisma Therapeutics, CureVac, BioNTech, Gilead, Tempest Therapeutics, Boehringer Ingelheim, Contracted Research: Regeneron Pharmaceutics, Dragonfly Therapeutics, Harbour Biomed Sciences, Ownership Interest Less Than 5 Percent: BreakBio, Apricity. JDB: Researcher: Merck, Genentech, Astrazeneca, Kite/Gilead, BMS, Celldex, Oncovir, Seattle Genetics, ADC Therapeutics, Epizyme, Consultant/Advisor/Speaker: Merck, Genentech, Astrazeneca, Kite/Gilead, BMS, Seattle Genetics, ADC Therapeutics, Epizyme, Asgaard Therapeutics, Global BioAccess, SIRPant Immunotherapeutics. JC: Executive Role: UofL Health, Researcher: Amgen, Iovance, Fate, Replimune, Consultant/Advisor/Speaker: Replimune advisory board 2020-21, Royalties or Patent Beneficiary: US Patents: University of Louisville. RC: Employee: Replimune. TdB: Consultant/Advisor/Speaker: Terumo, Boston Scientific, Astra Zeneca, Nanobiotix, Quantum Surgical, Guerbet, Cook medical, Johnson & Johnson. TDdG: Patents: (1) The use of cytostatics for the accelerated differentiation of DC; WO2009019320-A2; WO2009019320-A3; AU2008285598-A1; EP2281030-A2; CA2724018-A1; US2011117051-A1. US8,470,789B2; DCprime BV. (2) Immunoglobulins binding human Vγ9VÎ’2 T cell receptors; P31885NL00. Consulting Fees: Mendus (formerly Immunicum, formerly DCPrime BV), Partner Therapeutics, GE Health, LAVA Therapeutics, Contracted Research: Idera Pharmaceuticals, Macrophage Parma, Ownership Interest Less Than 5 Percent: LAVA Therapeutics. MGF: Employee: Regeneron. GVG: Employee: Merck. Publicly Traded Stocks: Immunogen, Aveo, Beta Bionics. KJH: Researcher: AstraZeneca, Boehringer-Ingelheim, Replimune, Consultant/Advisor/Speaker: Arch Oncology, AstraZeneca, BMS, Boehringer-Ingelheim, Codiak, Eisai, Inzen, Merck-Serono, MSD, Oncolys, Pfizer, Replimune. HK: Employee: Ankyra Therapeutics. Consultant/Advisor/Speaker: Castle Biosciences, Marengo Therapeutics. CMK: Researcher: Amgen, Merck & Co., Kartos Pharmaceuticals, EMD Serono, Deciphera Pharmaceuticals, Eily lilly, Blueprint Medicines, Incyte Corporation, Clovis Oncology, BMS, Aadi Bioscience, Pfizer, Iterion Therapeutics, Springworks Therapeutics, Nektar Therapeutics, GSK, Adaptimmune, Medimmune, Bioatla, Oncternal Therapeutics, Daiichi- Sankyo Co. Traycon Pharm, Eisai. Ascentage Pharma, Hutchison Medipharma, Salarius Pharmaceuticals, Loxo Oncology, -BTG Specialty Pharmaceuticals, AStex Pharmaceuticals, Xencor, bayer healthcare, Athenex pharmaceuticals, servier, K- group Beta, Trilliium Pharmaceuticals, Ayala Pharmaceuticals, Ningbo Newbay Technology Development, Rain Therapeutics, Inhibrx, C4 Therapeutics, Foghorn Therapeutics, Theseus Pharmaceuticals, Cogent Therapeutics, Curadev PharmaRegeneron, Consultant/Advisor/Speaker: Chemocentryx, Kartos, Servier, Immunicom, Other: Spouse employed by Daichii Sankyo with stock options. ADK: Employee: Merck. KL: Employee: Marengo Therapeutics. SL: Executive Role: Big Against Cancer (Belgium), Breast Cancer Trials (Australia), International Breast Cancer Study Group (Switzerland) Researcher: Research funding to institution from Novartis, Bristol Meyers Squibb, Merck, Puma Biotechnology, Eli Lilly, Nektar Therapeutics, Astra Zeneca, Roche-Genentech and Seattle Genetics, Consultant/Advisor/Speaker: Consultant (not compensated) to Seattle Genetics, Novartis, Bristol Meyers Squibb, Merck, AstraZeneca, Eli Lilly, Pfizer, Gilead Therapeutics and Roche-Genentech. Consultant (paid to institution) to Aduro Biotech, Novartis, GlaxoSmithKline, Roche-Genentech, Astra Zeneca, Silverback Therapeutics, G1 Therapeutics, PUMA Biotechnologies, Pfizer, Gilead Therapeutics, Seattle Genetics, Daiichi Sankyo, Merck, Amunix, Tallac Therapeutics, Eli Lilly and Bristol Meyers Squibb, Role: Presenter (Speaker). GVL: Consultant/Advisor/Speaker: Consultant Advisor for Agenus, Amgen, Array Biopharma, AstraZeneca UK, Boehringer Ingelheim International, Bristol Myers Squibb, Evaxion Biotech A/S, Hexal AG (Sandoz Company), Highlight Therapeutics S.L., Innovent Biologics USA, Merck Sharpe & Dohme (Australia), Merck Sharpe & Dohme, Novartis Pharma AG, PHMR, Pierre Fabre, Provectus Australia, Qbiotics Group Limited, Regeneron Pharmaceuticals. IM: Consulting Fees: Bristol-Myers Squibb, F-STAR, Alligator, Pharma Mar, AstraZeneca, Numab Therapeutics, Roche, Amunix, Gossamer, Molecular Partners, Merck-Serono, Genmab, PharmaMar, Contracted Research: Roche, Bristol-Myers Squibb, Highlight Therapeutics, Alligator, Genmab, Astrazeneca. MRM: Researcher : Roche, Astrazeneca, Novartis, Immunocore, BMS, Pfizer, Merck/MSD, Regeneron, BiolineRx, Replimune, GRAIL, Alkermes, iOx, Vaccitech, Consultant/Advisor/Speaker: Infinitopes. BN: Researcher: As a principal investigator for clinical trials sponsored by UZ Brussel, my institution received support from Novartis, Pfizer, Bayer, BMS, MSD, Pierre-Fabre, Amgen, Consultant/Advisor/Speaker: Novartis, BMS, MSD, Pfizer, Pierre-Fabre, Amgen. DJP: Consulting Fees: ViiV Healthcare, Bayer, Hoffman La Roche, EISAI, H3B, MiNa Alpha Therapeutics, DaVolterra, Fees for Non CE Services: Hoffmann La Roche, EISAI, Contracted Research: Merck Sharpe and Dohme, Bristol Myers Squibb (to institution). RAS: Researcher: Boston ScientificConsultant/Advisor/Speaker: Cook Medical, TriSalus, Replimune, Medtronic. SBS: Owner: Aperture Medical Technology, Executive Role: Aperture Medical Technology, Researcher: GE Healthcare, Johnson & Johnson, Elesta, Consultant/Advisor/Speaker: GE Healthcare, XACT Robotics, Microbot, Candel Therapeutics, Varian, Merck & Co., Royalty and Patent Beneficiary: Aperture Medical Technology, Publicly Traded Stocks: Johnson & Johnson, Poseidx Therapeutics, Motus GI, Sientra, Avadel, Lantheus. PS: Employee: Johnson & Johnson, Publicly Traded Stocks: Johnson & Johnson. SITC staff members SMW, CG, AK, NL, EG and KJ have no disclosures.
Figures
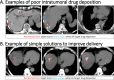
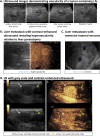
Similar articles
-
Review of the 25th annual scientific meeting of the International Society for Biological Therapy of Cancer.J Transl Med. 2011 May 12;9:60. doi: 10.1186/1479-5876-9-60. J Transl Med. 2011. PMID: 21569425 Free PMC article.
-
Society for Immunotherapy of Cancer (SITC) clinical practice guideline on immunotherapy for the treatment of urothelial cancer.J Immunother Cancer. 2021 Jul;9(7):e002552. doi: 10.1136/jitc-2021-002552. J Immunother Cancer. 2021. PMID: 34266883 Free PMC article.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Challenges and opportunities in cancer immunotherapy: a Society for Immunotherapy of Cancer (SITC) strategic vision.J Immunother Cancer. 2024 Jun 19;12(6):e009063. doi: 10.1136/jitc-2024-009063. J Immunother Cancer. 2024. PMID: 38901879 Free PMC article. Review.
-
Intratumoral Immunotherapy: From Trial Design to Clinical Practice.Clin Cancer Res. 2021 Feb 1;27(3):665-679. doi: 10.1158/1078-0432.CCR-20-0473. Epub 2020 Sep 17. Clin Cancer Res. 2021. PMID: 32943460 Review.
Cited by
-
An Abscopal Effect on Lung Metastases in Canine Mammary Cancer Patients Induced by Neoadjuvant Intratumoral Immunotherapy with Cowpea Mosaic Virus Nanoparticles and Anti-Canine PD-1.Cells. 2024 Sep 3;13(17):1478. doi: 10.3390/cells13171478. Cells. 2024. PMID: 39273048 Free PMC article.
-
The role of HGH1 in breast cancer prognosis: a study on immune response and cell cycle.BMC Cancer. 2024 Sep 9;24(1):1122. doi: 10.1186/s12885-024-12879-2. BMC Cancer. 2024. PMID: 39251967 Free PMC article.
References
-
- Idera Pharmaceuticals, Inc . Idera pharmaceuticals announces results from illuminate-301 trial of Tilsotolimod + Ipilimumab in anti-PD-1 refractory advanced Melanoma. news release; 2021.
-
- Oncosec Immunotherapies . Oncosec announces clinical data of the KEYNOTE-695 trial assessing TAVO-EP in combination with KEYTRUDA (Pembrolizumab) in patients with advanced melanoma refractory to anti-PD-1 treatment. press release; 2023.
MeSH terms
LinkOut - more resources
Full Text Sources
