Sensitive detection of pathological seeds of α-synuclein, tau and prion protein on solid surfaces
- PMID: 38640117
- PMCID: PMC11062561
- DOI: 10.1371/journal.ppat.1012175
Sensitive detection of pathological seeds of α-synuclein, tau and prion protein on solid surfaces
Abstract
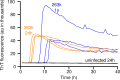
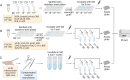
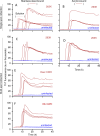
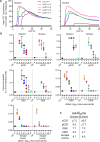
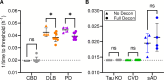
Prions or prion-like aggregates such as those composed of PrP, α-synuclein, and tau are key features of proteinopathies such as prion, Parkinson's and Alzheimer's diseases, respectively. Their presence on solid surfaces may be biohazardous under some circumstances. PrP prions bound to solids are detectable by ultrasensitive real-time quaking-induced conversion (RT-QuIC) assays if the solids can be immersed in assay wells or the prions transferred to pads. Here we show that prion-like seeds can remain detectable on steel wires for at least a year, or even after enzymatic cleaning and sterilization. We also show that contamination of larger objects with pathological seeds of α-synuclein, tau, and PrP can be detected by simply assaying a sampling medium that has been transiently applied to the surface. Human α-synuclein seeds in dementia with Lewy bodies brain tissue were detected by α-synuclein RT-QuIC after drying of tissue dilutions with concentrations as low as 10-6 onto stainless steel. Tau RT-QuIC detected tau seeding activity on steel exposed to Alzheimer's disease brain tissue diluted as much as a billion fold. Prion RT-QuIC assays detected seeding activity on plates exposed to brain dilutions as extreme as 10-5-10-8 from prion-affected humans, sheep, cattle and cervids. Sampling medium collected from surgical instruments used in necropsies of sporadic Creutzfeldt-Jakob disease-infected transgenic mice was positive down to 10-6 dilution. Sensitivity for prion detection was not sacrificed by omitting the recombinant PrP substrate from the sampling medium during its application to a surface and subsequent storage as long as the substrate was added prior to performing the assay reaction. Our findings demonstrate practical prototypic surface RT-QuIC protocols for the highly sensitive detection of pathologic seeds of α-synuclein, tau, and PrP on solid objects.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
BC, CDO, BG and AH are inventors on patent applications pertaining to RT-QuIC technology. The other authors have declared that no competing interests exist. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Government.
Figures



Similar articles
-
Ultrasensitive RT-QuIC Seed Amplification Assays for Disease-Associated Tau, α-Synuclein, and Prion Aggregates.Methods Mol Biol. 2019;1873:19-37. doi: 10.1007/978-1-4939-8820-4_2. Methods Mol Biol. 2019. PMID: 30341601
-
Defining the Protein Seeds of Neurodegeneration using Real-Time Quaking-Induced Conversion Assays.Biomolecules. 2020 Aug 25;10(9):1233. doi: 10.3390/biom10091233. Biomolecules. 2020. PMID: 32854212 Free PMC article. Review.
-
Prion-Like Seeding of Misfolded α-Synuclein in the Brains of Dementia with Lewy Body Patients in RT-QUIC.Mol Neurobiol. 2018 May;55(5):3916-3930. doi: 10.1007/s12035-017-0624-1. Epub 2017 May 26. Mol Neurobiol. 2018. PMID: 28550528 Free PMC article.
-
RT-QuIC and Related Assays for Detecting and Quantifying Prion-like Pathological Seeds of α-Synuclein.Biomolecules. 2022 Apr 14;12(4):576. doi: 10.3390/biom12040576. Biomolecules. 2022. PMID: 35454165 Free PMC article. Review.
-
Seed amplification and RT-QuIC assays to investigate protein seed structures and strains.Cell Tissue Res. 2023 Apr;392(1):323-335. doi: 10.1007/s00441-022-03595-z. Epub 2022 Mar 8. Cell Tissue Res. 2023. PMID: 35258712 Review.
Cited by
-
Rapid and sensitive determination of residual prion infectivity from prion-decontaminated surfaces.mSphere. 2024 Sep 25;9(9):e0050424. doi: 10.1128/msphere.00504-24. Epub 2024 Aug 27. mSphere. 2024. PMID: 39189773 Free PMC article.
-
Sodium hypochlorite inactivation of human CJD prions.PLoS One. 2024 Nov 7;19(11):e0312837. doi: 10.1371/journal.pone.0312837. eCollection 2024. PLoS One. 2024. PMID: 39509453 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

