CCAAT enhancer binding protein delta activates vesicle associated membrane protein 3 transcription to enhance chemoresistance and extracellular PD-L1 expression in triple-negative breast cancer
- PMID: 38627816
- PMCID: PMC11020785
- DOI: 10.1186/s13046-024-03041-8
CCAAT enhancer binding protein delta activates vesicle associated membrane protein 3 transcription to enhance chemoresistance and extracellular PD-L1 expression in triple-negative breast cancer
Abstract
Background: Chemoresistance and immunosuppression are two major obstacles in the current anti-cancer treatments. This study investigates the involvements of a CCAAT enhancer binding protein delta (CEBPD)/vesicle associated membrane protein 3 (VAMP3) axis in paclitaxel (PTX) resistance and immune evasion in triple-negative breast cancer (TNBC).
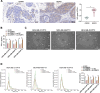
Methods: PTX resistance-related genes were screened by bioinformatics. CEBPD and VAMP3 expression in clinical TNBC samples was examined by immunohistochemistry. Three PTX-resistant TNBC cell lines (MDA-MB-231/PTX, MDA-MB-468/PTX and MDA-MB-453/PTX) were generated, and their drug resistance was analyzed. Autophagy of cells was analyzed by immunofluorescence staining. Interaction between CEBPD and VAMP3 promoter was identified by immunoprecipitation and luciferase assays. The extracellular expression of programmed cell death-ligand 1 (PD-L1) in TNBC cells was detected. Extracellular vesicles (EVs) from TNBC cells were isolated to examine their effects on CD8+ T cell exhaustion.
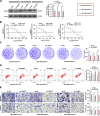
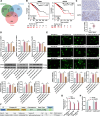
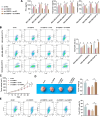
Results: CEBPD and VAMP3 were upregulated in chemo-resistant tissue samples and in PTX-resistant TNBC cells. The CEBPD downregulation enhanced PTX sensitivity of cells. However, further upregulation of VAMP3 in cells restored PTX resistance, which was likely due to the activation of autophagy, as the autophagy antagonist chloroquine enhanced PTX sensitivity of cells. CEBPD was found to bind to the VAMP3 promoter to activate its transcription. The CEBPD/VAMP3 axis also increased the PD-L1 expression in the conditioned medium of TNBC cells. The TNBC cell-derived EVs increased the exhaustion of co-cultured CD8+ T cells.
Conclusion: This study provides novel evidence that CEBPD plays a key role in enhancing PTX resistance in TNBC cells across various subtypes through VAMP3-mediated autophagy activation. Additionally, the CEBPD/VAMP3 axis also increases extracellular PD-L1 level, delivered by cancer cell-derived EVs, to suppress CD8+ T cell-mediated anti-tumor immune response. These significant observations may provide new insights into the treatment of TNBC, suggesting CEBPD and VAMP3 as promising targets to overcome treatment resistance.
Keywords: Autophagy; CEBPD; Immune evasion; Paclitaxel resistance; Triple-negative breast cancer; VAMP3.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Therapeutic impact of Nintedanib with paclitaxel and/or a PD-L1 antibody in preclinical models of orthotopic primary or metastatic triple negative breast cancer.J Exp Clin Cancer Res. 2019 Jan 11;38(1):16. doi: 10.1186/s13046-018-0999-5. J Exp Clin Cancer Res. 2019. PMID: 30635009 Free PMC article.
-
ALG3 predicts poor prognosis and increases resistance to anti-PD-1 therapy through modulating PD-L1 N-link glycosylation in TNBC.Int Immunopharmacol. 2024 Oct 25;140:112875. doi: 10.1016/j.intimp.2024.112875. Epub 2024 Aug 9. Int Immunopharmacol. 2024. PMID: 39116492
-
Low level of ARID1A contributes to adaptive immune resistance and sensitizes triple-negative breast cancer to immune checkpoint inhibitors.Cancer Commun (Lond). 2023 Sep;43(9):1003-1026. doi: 10.1002/cac2.12465. Epub 2023 Jul 11. Cancer Commun (Lond). 2023. PMID: 37434394 Free PMC article.
-
The potential role of cofilin-1 in promoting triple negative breast cancer (TNBC) metastasis via the extracellular vesicles (EVs).Transl Oncol. 2022 Jan;15(1):101247. doi: 10.1016/j.tranon.2021.101247. Epub 2021 Oct 19. Transl Oncol. 2022. PMID: 34678587 Free PMC article. Review.
-
Extracellular vesicle-mediated drug delivery in breast cancer theranostics.Discov Oncol. 2024 May 23;15(1):181. doi: 10.1007/s12672-024-01007-y. Discov Oncol. 2024. PMID: 38780753 Free PMC article. Review.
Cited by
-
Modulation of the Oncogenic LINE-1 Regulatory Network in Non-Small Cell Lung Cancer by Exosomal miRNAs.Int J Mol Sci. 2024 Oct 3;25(19):10674. doi: 10.3390/ijms251910674. Int J Mol Sci. 2024. PMID: 39409003 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

