Process-Based and Probabilistic Quantification of Co and Ni Mobilization Risks Induced by Managed Aquifer Recharge
- PMID: 38624010
- PMCID: PMC11064215
- DOI: 10.1021/acs.est.3c10583
Process-Based and Probabilistic Quantification of Co and Ni Mobilization Risks Induced by Managed Aquifer Recharge
Abstract
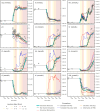
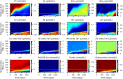
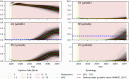
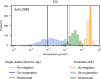
Managed aquifer recharge (MAR) is an increasingly used water management technique that enhances water availability while commonly generating water quality benefits. However, MAR activities may also trigger adverse geochemical reactions, especially during the injection of oxidant-enriched waters into reducing aquifers. Where this occurs, the environmental risks and the viability of mitigating them must be well understood. Here, we develop a rigorous approach for assessing and managing the risks from MAR-induced metal mobilization. First, we develop a process-based reactive transport model to identify and quantify the main hydrogeochemical drivers that control the release of metals and their mobility. We then apply a probabilistic framework to interrogate the inherent uncertainty associated with adjustable model parameters and consider this uncertainty (i) in long-term predictions of groundwater quality changes and (ii) in scenarios that investigate the effectiveness of modifications in the water treatment process to mitigate metal release and mobility. The results suggested that Co, Ni, Zn, and Mn were comobilized during pyrite oxidation and that metal mobility was controlled (i) by the sediment pH buffering capacity and (ii) by the sorption capacity of the native aquifer sediments. Both tested mitigation strategies were shown to be effective at reducing the risk of elevated metal concentrations.
Keywords: managed aquifer recharge; metal mobility; probabilistic framework; reactive transport modeling; uncertainty.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Mobilization of Arsenic and Other Naturally Occurring Contaminants during Managed Aquifer Recharge: A Critical Review.Environ Sci Technol. 2021 Feb 16;55(4):2208-2223. doi: 10.1021/acs.est.0c07492. Epub 2021 Jan 27. Environ Sci Technol. 2021. PMID: 33503373 Review.
-
Controlling Arsenic Mobilization during Managed Aquifer Recharge: The Role of Sediment Heterogeneity.Environ Sci Technol. 2020 Jul 21;54(14):8728-8738. doi: 10.1021/acs.est.0c00794. Epub 2020 Jul 6. Environ Sci Technol. 2020. PMID: 32516527
-
Geochemical Triggers of Arsenic Mobilization during Managed Aquifer Recharge.Environ Sci Technol. 2015 Jul 7;49(13):7802-9. doi: 10.1021/acs.est.5b01140. Epub 2015 Jun 25. Environ Sci Technol. 2015. PMID: 26057865
-
Fluoride release from carbonate-rich fluorapatite during managed aquifer recharge: Model-based development of mitigation strategies.Water Res. 2021 Apr 1;193:116880. doi: 10.1016/j.watres.2021.116880. Epub 2021 Jan 28. Water Res. 2021. PMID: 33578057
-
Managed Aquifer Recharge in Mining: A Review.Ground Water. 2023 May-Jun;61(3):305-317. doi: 10.1111/gwat.13311. Epub 2023 Apr 8. Ground Water. 2023. PMID: 36950867 Review.
References
-
- Dillon P.; Stuyfzand P.; Grischek T.; Lluria M.; Pyne R. D. G.; Jain R. C.; Bear J.; Schwarz J.; Wang W.; Fernandez E.; et al Sixty years of global progress in managed aquifer recharge. Hydrogeol. J. 2019, 27 (1), 1–30. 10.1007/s10040-018-1841-z. - DOI
-
- Vanderzalm J. L.; Dillon P. J.; Barry K. E.; Miotlinski K.; Kirby J. K. Arsenic mobility and impact on recovered water quality during aquifer storage and recovery using reclaimed water in a carbonate aquifer. Appl. Geochem. 2011, 26 (12), 1946–1955. 10.1016/j.apgeochem.2011.06.025. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

