Flagellin-modulated inflammasome pathways characterize the human alveolar macrophage response to Burkholderia pseudomallei, a lung-tropic pathogen
- PMID: 38619302
- PMCID: PMC11075458
- DOI: 10.1128/iai.00060-24
Flagellin-modulated inflammasome pathways characterize the human alveolar macrophage response to Burkholderia pseudomallei, a lung-tropic pathogen
Abstract
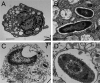
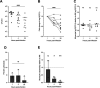
Melioidosis is an emerging tropical infection caused by inhalation, inoculation, or ingestion of the flagellated, facultatively intracellular pathogen Burkholderia pseudomallei. The melioidosis case fatality rate is often high, and pneumonia, the most common presentation, doubles the risk of death. The alveolar macrophage is a sentinel pulmonary host defense cell, but the human alveolar macrophage in B. pseudomallei infection has never been studied. The objective of this study was to investigate the host-pathogen interaction of B. pseudomallei infection with the human alveolar macrophage and to determine the role of flagellin in modulating inflammasome-mediated pathways. We found that B. pseudomallei infects primary human alveolar macrophages but is gradually restricted in the setting of concurrent cell death. Electron microscopy revealed cytosolic bacteria undergoing division, indicating that B. pseudomallei likely escapes the alveolar macrophage phagosome and may replicate in the cytosol, where it triggers immune responses. In paired human blood monocytes, uptake and intracellular restriction of B. pseudomallei are similar to those observed in alveolar macrophages, but cell death is reduced. The alveolar macrophage cytokine response to B. pseudomallei is characterized by marked interleukin (IL)-18 secretion compared to monocytes. Both cytotoxicity and IL-18 secretion in alveolar macrophages are partially flagellin dependent. However, the proportion of IL-18 release that is driven by flagellin is greater in alveolar macrophages than in monocytes. These findings suggest differential flagellin-mediated inflammasome pathway activation in the human alveolar macrophage response to B. pseudomallei infection and expand our understanding of intracellular pathogen recognition by this unique innate immune lung cell.
Keywords: Burkholderia pseudomallei; alveolar macrophage; flagella.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Burkholderia pseudomallei triggers canonical inflammasome activation in a human primary macrophage-based infection model.PLoS Negl Trop Dis. 2020 Nov 2;14(11):e0008840. doi: 10.1371/journal.pntd.0008840. eCollection 2020 Nov. PLoS Negl Trop Dis. 2020. PMID: 33137811 Free PMC article.
-
Flagellin-independent effects of a Toll-like receptor 5 polymorphism in the inflammatory response to Burkholderia pseudomallei.PLoS Negl Trop Dis. 2019 May 8;13(5):e0007354. doi: 10.1371/journal.pntd.0007354. eCollection 2019 May. PLoS Negl Trop Dis. 2019. PMID: 31067234 Free PMC article.
-
Burkholderia mallei and Burkholderia pseudomallei stimulate differential inflammatory responses from human alveolar type II cells (ATII) and macrophages.Front Cell Infect Microbiol. 2012 Dec 28;2:165. doi: 10.3389/fcimb.2012.00165. eCollection 2012. Front Cell Infect Microbiol. 2012. PMID: 23293773 Free PMC article.
-
Role of Canonical and Non-canonical Inflammasomes During Burkholderia Infection.Curr Top Microbiol Immunol. 2016;397:199-214. doi: 10.1007/978-3-319-41171-2_10. Curr Top Microbiol Immunol. 2016. PMID: 27460811 Review.
-
Immunity to Burkholderia pseudomallei.Curr Opin Infect Dis. 2009 Apr;22(2):102-8. doi: 10.1097/QCO.0b013e328322e727. Curr Opin Infect Dis. 2009. PMID: 19276877 Review.
References
-
- CDC . 2022. Melioidosis locally endemic in areas of the Mississippi Gulf coast after Burkholderia pseudomallei isolated in soil and water and linked to two cases – Mississippi, 2020 and 2022. Available from: https://emergency.cdc.gov/han/2022/han00470.asp. Retrieved 13 Jan 2023.
-
- Chantratita N, Phunpang R, Yarasai A, Dulsuk A, Yimthin T, Onofrey LA, Coston TD, Thiansukhon E, Chaisuksant S, Tanwisaid K, Chuananont S, Morakot C, Sangsa N, Chayangsu S, Silakun W, Buasi N, Chetchotisakd P, Day NP, Lertmemongkolchai G, West TE. 2023. Characteristics and one year outcomes of melioidosis patients in northeastern Thailand: a prospective, multicenter cohort study. Lancet Reg Health Southeast Asia 9:100118. doi:10.1016/j.lansea.2022.100118 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

