Underlying Mechanisms behind the Brain-Gut-Liver Axis and Metabolic-Associated Fatty Liver Disease (MAFLD): An Update
- PMID: 38612504
- PMCID: PMC11011299
- DOI: 10.3390/ijms25073694
Underlying Mechanisms behind the Brain-Gut-Liver Axis and Metabolic-Associated Fatty Liver Disease (MAFLD): An Update
Abstract
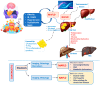
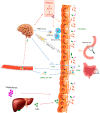
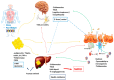
Metabolic-associated fatty liver disease (MAFLD) includes several metabolic dysfunctions caused by dysregulation in the brain-gut-liver axis and, consequently, increases cardiovascular risks and fatty liver dysfunction. In MAFLD, type 2 diabetes mellitus, obesity, and metabolic syndrome are frequently present; these conditions are related to liver lipogenesis and systemic inflammation. This study aimed to review the connection between the brain-gut-liver axis and MAFLD. The inflammatory process, cellular alterations in hepatocytes and stellate cells, hypercaloric diet, and sedentarism aggravate the prognosis of patients with MAFLD. Thus, to understand the modulation of the physiopathology of MAFLD, it is necessary to include the organokines involved in this process (adipokines, myokines, osteokines, and hepatokines) and their clinical relevance to project future perspectives of this condition and bring to light new possibilities in therapeutic approaches. Adipokines are responsible for the activation of distinct cellular signaling in different tissues, such as insulin and pro-inflammatory cytokines, which is important for balancing substances to avoid MAFLD and its progression. Myokines improve the quantity and quality of adipose tissues, contributing to avoiding the development of MAFLD. Finally, hepatokines are decisive in improving or not improving the progression of this disease through the regulation of pro-inflammatory and anti-inflammatory organokines.
Keywords: MAFLD; brain–gut–liver axis; diabetes; dyslipidemia; insulin resistance; metabolic-associated fatty liver disease; obesity; organokines.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
Non-Alcoholic Steatohepatitis (NASH) and Organokines: What Is Now and What Will Be in the Future.Int J Mol Sci. 2022 Jan 2;23(1):498. doi: 10.3390/ijms23010498. Int J Mol Sci. 2022. PMID: 35008925 Free PMC article. Review.
-
The gut-liver axis: emerging mechanisms and therapeutic approaches for nonalcoholic fatty liver disease and type 2 diabetes mellitus.Naunyn Schmiedebergs Arch Pharmacol. 2024 Nov;397(11):8421-8443. doi: 10.1007/s00210-024-03204-6. Epub 2024 Jun 11. Naunyn Schmiedebergs Arch Pharmacol. 2024. PMID: 38861011 Review.
-
Metabolic dysfunction-associated fatty liver disease is an early predictor for testosterone deficiency in aging men without metabolic syndrome.Front Endocrinol (Lausanne). 2023 Oct 3;14:1252774. doi: 10.3389/fendo.2023.1252774. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37854195 Free PMC article.
-
MAFLD as part of systemic metabolic dysregulation.Hepatol Int. 2024 Oct;18(Suppl 2):834-847. doi: 10.1007/s12072-024-10660-y. Epub 2024 Apr 9. Hepatol Int. 2024. PMID: 38594474 Review.
-
Organokines in disease.Adv Clin Chem. 2020;94:261-321. doi: 10.1016/bs.acc.2019.07.012. Epub 2019 Aug 12. Adv Clin Chem. 2020. PMID: 31952573 Review.
Cited by
-
Metabolic-Associated Fatty Liver Disease: The Influence of Oxidative Stress, Inflammation, Mitochondrial Dysfunctions, and the Role of Polyphenols.Pharmaceuticals (Basel). 2024 Oct 10;17(10):1354. doi: 10.3390/ph17101354. Pharmaceuticals (Basel). 2024. PMID: 39458995 Free PMC article. Review.
References
-
- Krolenko E.V., Kupriyanova O.V., Nigmatullina L.S., Grigoryeva T.V., Roumiantsev S.A., Shestopalov A.V. Changes of the Concentration of Short-Chain Fatty Acids in the Intestines of Mice with Different Types of Obesity. Bull. Exp. Biol. Med. 2024;176:347–353. doi: 10.1007/s10517-024-06022-1. - DOI - PubMed
-
- Carpi R.Z., Barbalho S.M., Sloan K.P., Laurindo L.F., Gonzaga H.F., Grippa P.C., Zutin T.L.M., Girio R.J.S., Repetti C.S.F., Detregiachi C.R.P., et al. The Effects of Probiotics, Prebiotics and Synbiotics in Non-Alcoholic Fat Liver Disease (NAFLD) and Non-Alcoholic Steatohepatitis (NASH): A Systematic Review. Int. J. Mol. Sci. 2022;23:8805. doi: 10.3390/ijms23158805. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

