The Role of Natural Killer Cells in the Tumor Immune Microenvironment of EBV-Associated Nasopharyngeal Carcinoma
- PMID: 38610990
- PMCID: PMC11011204
- DOI: 10.3390/cancers16071312
The Role of Natural Killer Cells in the Tumor Immune Microenvironment of EBV-Associated Nasopharyngeal Carcinoma
Abstract
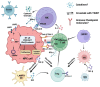
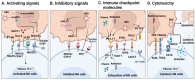
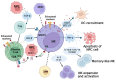
Endemic nasopharyngeal carcinoma (NPC) is closely associated with the Epstein-Barr virus (EBV), which contributes to tumor development and influences the tumor immune microenvironment (TIME) in NPC. Natural killer (NK) cells, as part of the innate immune system, play a crucial role in responding to viral infections and malignant cell transformations. Notably, NK cells possess a unique ability to target tumor cells independent of major histocompatibility complex class I (MHC I) expression. This means that MHC I-deficient tumor cells, which can escape from effective T cell attack, are susceptible to NK-cell-mediated killing. The activation of NK cells is determined by the signals generated through inhibitory and activating receptors expressed on their surface. Understanding the role of NK cells in the complex TIME of EBV+ NPC is of utmost importance. In this review, we provide a comprehensive summary of the current understanding of NK cells in NPC, focusing on their subpopulations, interactions, and cytotoxicity within the TIME. Moreover, we discuss the potential translational therapeutic applications of NK cells in NPC. This review aims to enhance our knowledge of the role of NK cells in NPC and provide valuable insights for future investigations.
Keywords: Epstein–Barr virus; anti-cancer therapy; nasopharyngeal carcinoma; natural killer cells; tumor immune microenvironment.
Conflict of interest statement
The author declares no conflicts of interest.
Figures
Similar articles
-
Comprehensive single-cell transcriptomic and proteomic analysis reveals NK cell exhaustion and unique tumor cell evolutionary trajectory in non-keratinizing nasopharyngeal carcinoma.J Transl Med. 2023 Apr 25;21(1):278. doi: 10.1186/s12967-023-04112-8. J Transl Med. 2023. PMID: 37098551 Free PMC article.
-
EBV-Upregulated B7-H3 Inhibits NK cell-Mediated Antitumor Function and Contributes to Nasopharyngeal Carcinoma Progression.Cancer Immunol Res. 2023 Jun 2;11(6):830-846. doi: 10.1158/2326-6066.CIR-22-0374. Cancer Immunol Res. 2023. PMID: 36996321
-
Epstein-Barr virus-encoded microRNA BART7 downregulates major histocompatibility complex class I chain-related peptide A and reduces the cytotoxicity of natural killer cells to nasopharyngeal carcinoma.Oncol Lett. 2018 Sep;16(3):2887-2892. doi: 10.3892/ol.2018.9041. Epub 2018 Jun 28. Oncol Lett. 2018. PMID: 30127876 Free PMC article.
-
The Role of NK Cells in EBV Infection and EBV-Associated NPC.Viruses. 2021 Feb 15;13(2):300. doi: 10.3390/v13020300. Viruses. 2021. PMID: 33671917 Free PMC article. Review.
-
Immunotherapeutic approaches in EBV-associated nasopharyngeal carcinoma.Front Immunol. 2023 Jan 11;13:1079515. doi: 10.3389/fimmu.2022.1079515. eCollection 2022. Front Immunol. 2023. PMID: 36713430 Free PMC article. Review.
Cited by
-
Automatic Annotation Diagnostic Framework for Nasopharyngeal Carcinoma via Pathology-Fidelity GAN and Prior-Driven Classification.Bioengineering (Basel). 2024 Jul 22;11(7):739. doi: 10.3390/bioengineering11070739. Bioengineering (Basel). 2024. PMID: 39061821 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

