Trivalent and quadrivalent seasonal influenza vaccine in adults aged 60 and older: a systematic review and network meta-analysis
- PMID: 38604619
- PMCID: PMC11287607
- DOI: 10.1136/bmjebm-2023-112767
Trivalent and quadrivalent seasonal influenza vaccine in adults aged 60 and older: a systematic review and network meta-analysis
Abstract
Objectives: To compare the efficacy of influenza vaccines of any valency for adults 60 years and older.
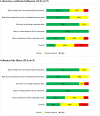
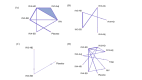
Design and setting: Systematic review with network meta-analysis (NMA) of randomised controlled trials (RCTs). MEDLINE, EMBASE, JBI Evidence-Based Practice (EBP) Database, PsycINFO, and Cochrane Evidence -Based Medicine database were searched from inception to 20 June 20, 2022. Two reviewers screened, abstracted, and appraised articles (Cochrane Risk of Bias (ROB) 2.0 tool) independently. We assessed certainty of findings using Confidence in Network Meta-Analysis and Grading of Recommendations, Assessment, Development and Evaluations approaches. We performed random-effects meta-analysis and network meta-analysis (NMA), and estimated odds ratios (ORs) for dichotomous outcomes and incidence rate ratios (IRRs) for count outcomes along with their corresponding 95% confidence intervals (CIs) and prediction intervals.
Participants: Older adults (≥60 years old) receiving an influenza vaccine licensed in Canada or the USA (vs placebo, no vaccine, or any other licensed vaccine), at any dose.
Main outcome measures: Laboratory-confirmed influenza (LCI) and influenza-like illness (ILI). Secondary outcomes were the number of vascular adverse events, hospitalisation for acute respiratory infection (ARI) and ILI, inpatient hospitalisation, emergency room (ER) visit for ILI, outpatient visit, and mortality, among others.
Results: We included 41 RCTs and 15 companion reports comprising 8 vaccine types and 206 032 participants. Vaccines may prevent LCI compared with placebo, with high-dose trivalent inactivated influenza vaccine (IIV3-HD) (NMA: 9 RCTs, 52 202 participants, OR 0.23, 95% confidence interval (CI) (0.11 to 0.51), low certainty of evidence) and recombinant influenza vaccine (RIV) (OR 0.25, 95%CI (0.08 to 0.73), low certainty of evidence) among the most efficacious vaccines. Standard dose trivalent IIV3 (IIV3-SD) may prevent ILI compared with placebo, but the result was imprecise (meta-analysis: 2 RCTs, 854 participants, OR 0.39, 95%CI (0.15 to 1.02), low certainty of evidence). Any HD was associated with prevention of ILI compared with placebo (NMA: 9 RCTs, 65 658 participants, OR 0.38, 95%CI (0.15 to 0.93)). Adjuvanted quadrivalent IIV (IIV4-Adj) may be associated with the least vascular adverse events, but the results were very uncertain (NMA: eight 8 RCTs, 57 677 participants, IRR 0.18, 95%CI (0.07 to 0.43), very low certainty of evidence). RIV on all-cause mortality may be comparable to placebo (NMA: 20 RCTs, 140 577 participants, OR 1.01, 95%CI (0.23 to 4.49), low certainty of evidence).
Conclusions: This systematic review demonstrated efficacy associated with IIV3-HD and RIV vaccines in protecting older persons against LCI. RIV vaccine may reduce all-cause mortality when compared with other vaccines, but the evidence is uncertain. Differences in efficacy between influenza vaccines remain uncertain with very low to moderate certainty of evidence.
Prospero registration number: CRD42020177357.
Keywords: immunization.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

Similar articles
-
Comparative efficacy and safety of vaccines to prevent seasonal influenza: A systematic review and network meta-analysis.EClinicalMedicine. 2022 Mar 25;46:101331. doi: 10.1016/j.eclinm.2022.101331. eCollection 2022 Apr. EClinicalMedicine. 2022. PMID: 35360146 Free PMC article.
-
Vaccines for preventing influenza in the elderly.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004876. doi: 10.1002/14651858.CD004876.pub4. Cochrane Database Syst Rev. 2018. PMID: 29388197 Free PMC article. Review.
-
Vaccines for preventing influenza in healthy adults.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD001269. doi: 10.1002/14651858.CD001269.pub6. Cochrane Database Syst Rev. 2018. PMID: 29388196 Free PMC article. Review.
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Vaccines for preventing influenza in healthy children.Cochrane Database Syst Rev. 2018 Feb 1;2(2):CD004879. doi: 10.1002/14651858.CD004879.pub5. Cochrane Database Syst Rev. 2018. PMID: 29388195 Free PMC article. Review.
Cited by
-
Summary of the National Advisory Committee on Immunization (NACI) Supplemental Guidance on Influenza Vaccination in Adults 65 Years of Age and Older.Can Commun Dis Rep. 2024 Nov 7;50(11):387-392. doi: 10.14745/ccdr.v50i11a02. eCollection 2024 Nov. Can Commun Dis Rep. 2024. PMID: 39525077 Free PMC article.
References
-
- Canada Go . Flu (influenza): for health professionals. 2023.
-
- Ng C, Ye L, Noorduyn SG, et al. . Resource utilization and cost of influenza requiring hospitalization in Canadian adults: A study from the serious outcomes surveillance network of the Canadian immunization research network. Influenza Other Respir Viruses 2018;12:232–40. 10.1111/irv.12521 - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention . Key facts about influenza (flu). 2019. Atlanta, Georgia: Centers for Disease Control and Prevention, 2021.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
