Nanobodies against African swine fever virus p72 and CD2v proteins as reagents for developing two cELISAs to detect viral antibodies
- PMID: 38588947
- PMCID: PMC11280129
- DOI: 10.1016/j.virs.2024.04.002
Nanobodies against African swine fever virus p72 and CD2v proteins as reagents for developing two cELISAs to detect viral antibodies
Abstract
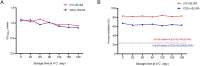
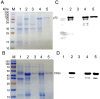
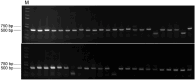
African swine fever virus (ASFV) poses a significant threat to the global swine industry. Currently, there are no effective vaccines or treatments available to combat ASFV infection in pigs. The primary means of controlling the spread of the disease is through rapid detection and subsequent elimination of infected pig. Recently, a lower virulent ASFV isolate with a deleted EP402R gene (CD2v-deleted) has been reported in China, which further complicates the control of ASFV infection in pig farms. Furthermore, an EP402R-deleted ASFV variant has been developed as a potential live attenuated vaccine candidate strain. Therefore, it is crucial to develop detection methods that can distinguish wild-type and EP402R-deleted ASFV infections. In this study, two recombinant ASFV-p72 and -CD2v proteins were expressed using a prokaryotic system and used to immunize Bactrian camels. Subsequently, eight nanobodies against ASFV-p72 and ten nanobodies against ASFV-CD2v were screened. Following the production of these nanobodies with horse radish peroxidase (HRP) fusion proteins, the ASFV-p72-Nb2-HRP and ASFV-CD2v-Nb22-HRP fusions were selected for the development of two competitive ELISAs (cELISAs) to detect anti-ASFV antibodies. The two cELISAs exhibited high sensitivity, good specificity, repeatability, and stability. The coincidence rate between the two cELISAs and commercial ELISA kits was 98.6% and 97.6%, respectively. Collectively, the two cELISA for detecting antibodies against ASFV demonstrated ease of operation, a low cost, and a simple production process. The two cELISAs could determine whether pigs were infected with wild-type or CD2v-deleted ASFV, and could play an important role in monitoring ASFV infections in pig farms.
Keywords: ASFV-CD2v; ASFV-p72; African swine fever virus (ASFV); Competitive ELISA; Nanobody-HRP.
Copyright © 2024 The Authors. Publishing services by Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflict of interest The authors report no conflicts of interest in this work.
Figures
Similar articles
-
A simple nanobody-based competitive ELISA to detect antibodies against African swine fever virus.Virol Sin. 2022 Dec;37(6):922-933. doi: 10.1016/j.virs.2022.09.004. Epub 2022 Sep 8. Virol Sin. 2022. PMID: 36089216 Free PMC article.
-
Development of an ELISA Method to Differentiate Animals Infected with Wild-Type African Swine Fever Viruses and Attenuated HLJ/18-7GD Vaccine Candidate.Viruses. 2022 Aug 6;14(8):1731. doi: 10.3390/v14081731. Viruses. 2022. PMID: 36016353 Free PMC article.
-
Epitope mapping and establishment of a blocking ELISA for mAb targeting the p72 protein of African swine fever virus.Appl Microbiol Biotechnol. 2024 May 29;108(1):350. doi: 10.1007/s00253-024-13146-x. Appl Microbiol Biotechnol. 2024. PMID: 38809284 Free PMC article.
-
African swine fever virus proteins involved in evading host defence systems.Vet Immunol Immunopathol. 2004 Aug;100(3-4):117-34. doi: 10.1016/j.vetimm.2004.04.002. Vet Immunol Immunopathol. 2004. PMID: 15207450 Review.
-
Host immune responses against African swine fever virus: Insights and challenges for vaccine development.Open Vet J. 2023 Dec;13(12):1517-1535. doi: 10.5455/OVJ.2023.v13.i12.2. Epub 2023 Dec 31. Open Vet J. 2023. PMID: 38292721 Free PMC article. Review.
References
-
- Biagetti M., Cuccioloni M., Bonfili L., Cecarini V., Sebastiani C., Curcio L., Giammarioli M., De Mia G.M., Eleuteri A.M., Angeletti M. Chimeric DNA/LNA-based biosensor for the rapid detection of African swine fever virus. Talanta. 2018;184:35–41. - PubMed
-
- Boinas F.S., Hutchings G.H., Dixon L.K., Wilkinson P.J. Characterization of pathogenic and non-pathogenic African swine fever virus isolates from Ornithodoros erraticus inhabiting pig premises in Portugal. J. Gen. Virol. 2004;85:2177–2187. - PubMed
-
- Burmakina G., Malogolovkin A., Tulman E.R., Zsak L., Delhon G., Diel D.G., Shobogorov N.M., Morgunov Y.P., Morgunov S.Y., Kutish G.F., Kolbasov D., Rock D.L. African swine fever virus serotype-specific proteins are significant protective antigens for African swine fever. J. Gen. Virol. 2016;97:1670–1675. - PubMed
-
- Caixia W., Songyin Q., Ying X., Haoyang Y., Haoxuan L., Shaoqiang W., Chunyan F., Xiangmei L. Development of a blocking ELISA kit for detection of ASFV antibody based on a monoclonal antibody against full-length p72. J. AOAC Int. 2022;105:1428–1436. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

