Hibiscus manihot L. flower extract induces anticancer activity through modulation of apoptosis and autophagy in A549 cells
- PMID: 38582921
- PMCID: PMC10998869
- DOI: 10.1038/s41598-024-58439-3
Hibiscus manihot L. flower extract induces anticancer activity through modulation of apoptosis and autophagy in A549 cells
Abstract
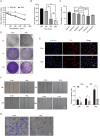
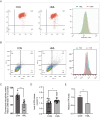
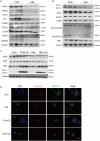
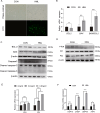
Lung cancer is a major public health issue and heavy burden in China and worldwide due to its high incidence and mortality without effective treatment. It's imperative to develop new treatments to overcome drug resistance. Natural products from food source, given their wide-ranging and long-term benefits, have been increasingly used in tumor prevention and treatment. This study revealed that Hibiscus manihot L. flower extract (HML) suppressed the proliferation and migration of A549 cells in a dose and time dependent manner and disrupting cell cycle progression. HML markedly enhanced the accumulation of ROS, stimulated the dissipation of mitochondrial membrane potential (MMP) and that facilitated mitophagy through the loss of mitochondrial function. In addition, HML induced apoptosis by activation of the PTEN-P53 pathway and inhibition of ATG5/7-dependent autophagy induced by PINK1-mediated mitophagy in A549 cells. Moreover, HML exert anticancer effects together with 5-FU through synergistic effect. Taken together, HML may serve as a potential tumor prevention and adjuvant treatment for its functional attributes.
Keywords: Hibiscus Manihot L flower; A549 cell; Apoptosis; Mitophagy; ROS.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Cedrol induces autophagy and apoptotic cell death in A549 non-small cell lung carcinoma cells through the P13K/Akt signaling pathway, the loss of mitochondrial transmembrane potential and the generation of ROS.Int J Mol Med. 2016 Jul;38(1):291-9. doi: 10.3892/ijmm.2016.2585. Epub 2016 May 11. Int J Mol Med. 2016. PMID: 27177023
-
Hibiscus flower extract selectively induces apoptosis in breast cancer cells and positively interacts with common chemotherapeutics.BMC Complement Altern Med. 2019 May 6;19(1):98. doi: 10.1186/s12906-019-2505-9. BMC Complement Altern Med. 2019. PMID: 31060537 Free PMC article.
-
PINK1 depletion sensitizes non-small cell lung cancer to glycolytic inhibitor 3-bromopyruvate: Involvement of ROS and mitophagy.Pharmacol Rep. 2019 Dec;71(6):1184-1189. doi: 10.1016/j.pharep.2019.08.002. Epub 2019 Aug 14. Pharmacol Rep. 2019. PMID: 31669882
-
Plumbagin promotes mitochondrial mediated apoptosis in gefitinib sensitive and resistant A549 lung cancer cell line through enhancing reactive oxygen species generation.Mol Biol Rep. 2020 Jun;47(6):4155-4168. doi: 10.1007/s11033-020-05464-w. Epub 2020 May 22. Mol Biol Rep. 2020. PMID: 32444975
-
Hsp90 inhibitor gedunin causes apoptosis in A549 lung cancer cells by disrupting Hsp90:Beclin-1:Bcl-2 interaction and downregulating autophagy.Life Sci. 2020 Sep 1;256:118000. doi: 10.1016/j.lfs.2020.118000. Epub 2020 Jun 22. Life Sci. 2020. PMID: 32585246
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

