RevCAR-expressing immune effector cells for targeting of Fn14-positive glioblastoma
- PMID: 38582787
- PMCID: PMC11405279
- DOI: 10.1038/s41417-024-00766-8
RevCAR-expressing immune effector cells for targeting of Fn14-positive glioblastoma
Abstract
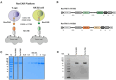
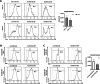
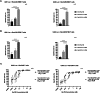
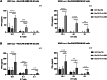
In recent studies, we have established the unique adapter chimeric antigen receptor (CAR) platform RevCAR which uses, as an extracellular CAR domain, a peptide epitope instead of an antibody domain. RevCAR adapters (termed RevCAR target modules, RevTMs) are bispecific antibodies that enable the reversible ON/OFF switch of the RevCAR system, improving the safety compared to conventional CARs. Here, we describe for the first time its use for retargeting of both T and NK-92 cells. In addition, we describe the development and preclinical validation of a novel RevTM for targeting of the fibroblast growth factor-inducible 14 (Fn14) surface receptor which is overexpressed on Glioblastoma (GBM) cells, and therefore serves as a promising target for the treatment of GBM. The novel RevTM efficiently redirects RevCAR modified T and NK-92 cells and leads to the killing of GBM cells both in vitro and in vivo. Tumor cell killing is associated with increased IL-2, TNF-α and/or IFN-γ secretion. Hence, these findings give an insight into the complementary potential of both RevCAR T and NK-92 systems as a safe and specific immunotherapeutic approach against GBM.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests. The research was partially funded by the “Europäischer Fonds für regionale Entwicklung (EFRE)”. This project is co-financed by tax funds based on the budget passed by the Saxon State Parliament (SAB-project number 100544183 to AF). CA is a fellow of the Mildred Scheel Early Career Center Dresden P2 funded by the German Cancer Aid (Deutsche Krebshilfe).
Figures


Similar articles
-
Specific and safe targeting of glioblastoma using switchable and logic-gated RevCAR T cells.Front Immunol. 2023 Apr 14;14:1166169. doi: 10.3389/fimmu.2023.1166169. eCollection 2023. Front Immunol. 2023. PMID: 37122703 Free PMC article.
-
CD19 CAR-expressing iPSC-derived NK cells effectively enhance migration and cytotoxicity into glioblastoma by targeting to the pericytes in tumor microenvironment.Biomed Pharmacother. 2024 May;174:116436. doi: 10.1016/j.biopha.2024.116436. Epub 2024 Mar 19. Biomed Pharmacother. 2024. PMID: 38508081
-
IL-13Rα2/TGF-β bispecific CAR-T cells counter TGF-β-mediated immune suppression and potentiate anti-tumor responses in glioblastoma.Neuro Oncol. 2024 Oct 3;26(10):1850-1866. doi: 10.1093/neuonc/noae126. Neuro Oncol. 2024. PMID: 38982561
-
CAR T-cell therapy for glioblastoma: recent clinical advances and future challenges.Neuro Oncol. 2018 Oct 9;20(11):1429-1438. doi: 10.1093/neuonc/noy032. Neuro Oncol. 2018. PMID: 29509936 Free PMC article. Review.
-
Current progress in chimeric antigen receptor T cell therapy for glioblastoma multiforme.Cancer Med. 2021 Aug;10(15):5019-5030. doi: 10.1002/cam4.4064. Epub 2021 Jun 19. Cancer Med. 2021. PMID: 34145792 Free PMC article. Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

