Functionally diverse thymic medullary epithelial cells interplay to direct central tolerance
- PMID: 38581680
- PMCID: PMC11079940
- DOI: 10.1016/j.celrep.2024.114072
Functionally diverse thymic medullary epithelial cells interplay to direct central tolerance
Abstract
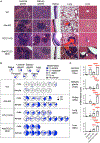
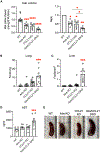
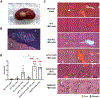
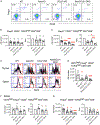
Medullary thymic epithelial cells (mTECs) are essential for the establishment of self-tolerance in T cells. Promiscuous gene expression by a subpopulation of mTECs regulated by the nuclear protein Aire contributes to the display of self-genomic products to newly generated T cells. Recent reports have highlighted additional self-antigen-displaying mTEC subpopulations, namely Fezf2-expressing mTECs and a mosaic of self-mimetic mTECs including thymic tuft cells. In addition, a functionally different subset of mTECs produces chemokine CCL21, which attracts developing thymocytes to the medullary region. Here, we report that CCL21+ mTECs and Aire+ mTECs non-redundantly cooperate to direct self-tolerance to prevent autoimmune pathology by optimizing the deletion of self-reactive T cells and the generation of regulatory T cells. We also detect cooperation for self-tolerance between Aire and Fezf2, the latter of which unexpectedly regulates thymic tuft cells. Our results indicate an indispensable interplay among functionally diverse mTECs for the establishment of central self-tolerance.
Keywords: Aire; CCL21; CP: Immunology; Fezf2; autoimmunity; central tolerance; medullary thymic epithelial cell; negative selection; regulatory T cell; thymic tuft cell; thymus medulla.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Similar articles
-
Developmental conversion of thymocyte-attracting cells into self-antigen-displaying cells in embryonic thymus medulla epithelium.Elife. 2024 Mar 11;12:RP92552. doi: 10.7554/eLife.92552. Elife. 2024. PMID: 38466627 Free PMC article.
-
Insm1 regulates mTEC development and immune tolerance.Cell Mol Immunol. 2023 Dec;20(12):1472-1486. doi: 10.1038/s41423-023-01102-0. Epub 2023 Nov 21. Cell Mol Immunol. 2023. PMID: 37990032 Free PMC article.
-
Novel antigen-presenting cell imparts Treg-dependent tolerance to gut microbiota.Nature. 2022 Oct;610(7933):752-760. doi: 10.1038/s41586-022-05309-5. Epub 2022 Sep 7. Nature. 2022. PMID: 36070798 Free PMC article.
-
Serial progression of cortical and medullary thymic epithelial microenvironments.Eur J Immunol. 2014 Jan;44(1):16-22. doi: 10.1002/eji.201344110. Epub 2013 Dec 4. Eur J Immunol. 2014. PMID: 24214487 Free PMC article. Review.
-
Thymic Mimetic Cells: Ontogeny as Immunology.Annu Rev Cell Dev Biol. 2024 Oct;40(1):283-300. doi: 10.1146/annurev-cellbio-112122-023316. Epub 2024 Sep 21. Annu Rev Cell Dev Biol. 2024. PMID: 38608315 Review.
Cited by
-
Generation and repair of thymic epithelial cells.J Exp Med. 2024 Oct 7;221(10):e20230894. doi: 10.1084/jem.20230894. Epub 2024 Jul 9. J Exp Med. 2024. PMID: 38980292 Free PMC article. Review.
-
Developmental conversion of thymocyte-attracting cells into self-antigen-displaying cells in embryonic thymus medulla epithelium.Elife. 2024 Mar 11;12:RP92552. doi: 10.7554/eLife.92552. Elife. 2024. PMID: 38466627 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

