Methodological biases in observational hospital studies of COVID-19 treatment effectiveness: pitfalls and potential
- PMID: 38576716
- PMCID: PMC10991758
- DOI: 10.3389/fmed.2024.1362192
Methodological biases in observational hospital studies of COVID-19 treatment effectiveness: pitfalls and potential
Abstract
Introduction: This study aims to discuss and assess the impact of three prevalent methodological biases: competing risks, immortal-time bias, and confounding bias in real-world observational studies evaluating treatment effectiveness. We use a demonstrative observational data example of COVID-19 patients to assess the impact of these biases and propose potential solutions.
Methods: We describe competing risks, immortal-time bias, and time-fixed confounding bias by evaluating treatment effectiveness in hospitalized patients with COVID-19. For our demonstrative analysis, we use observational data from the registry of patients with COVID-19 who were admitted to the Bellvitge University Hospital in Spain from March 2020 to February 2021 and met our predefined inclusion criteria. We compare estimates of a single-dose, time-dependent treatment with the standard of care. We analyze the treatment effectiveness using common statistical approaches, either by ignoring or only partially accounting for the methodological biases. To address these challenges, we emulate a target trial through the clone-censor-weight approach.
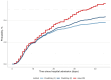
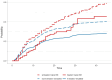
Results: Overlooking competing risk bias and employing the naïve Kaplan-Meier estimator led to increased in-hospital death probabilities in patients with COVID-19. Specifically, in the treatment effectiveness analysis, the Kaplan-Meier estimator resulted in an in-hospital mortality of 45.6% for treated patients and 59.0% for untreated patients. In contrast, employing an emulated trial framework with the weighted Aalen-Johansen estimator, we observed that in-hospital death probabilities were reduced to 27.9% in the "X"-treated arm and 40.1% in the non-"X"-treated arm. Immortal-time bias led to an underestimated hazard ratio of treatment.
Conclusion: Overlooking competing risks, immortal-time bias, and confounding bias leads to shifted estimates of treatment effects. Applying the naïve Kaplan-Meier method resulted in the most biased results and overestimated probabilities for the primary outcome in analyses of hospital data from COVID-19 patients. This overestimation could mislead clinical decision-making. Both immortal-time bias and confounding bias must be addressed in assessments of treatment effectiveness. The trial emulation framework offers a potential solution to address all three methodological biases.
Keywords: COVID-19; competing risks; confounding; emulated trial; immortal-time bias; methodological bias; treatment effectiveness.
Copyright © 2024 Martinuka, Hazard, Marateb, Mansourian, Mañanas, Romero, Rubio-Rivas and Wolkewitz.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Target trial emulation with multi-state model analysis to assess treatment effectiveness using clinical COVID-19 data.BMC Med Res Methodol. 2023 Sep 2;23(1):197. doi: 10.1186/s12874-023-02001-8. BMC Med Res Methodol. 2023. PMID: 37660025 Free PMC article.
-
Target Trial Emulation Using Hospital-Based Observational Data: Demonstration and Application in COVID-19.Life (Basel). 2023 Mar 13;13(3):777. doi: 10.3390/life13030777. Life (Basel). 2023. PMID: 36983933 Free PMC article.
-
Methodological evaluation of bias in observational coronavirus disease 2019 studies on drug effectiveness.Clin Microbiol Infect. 2021 Jul;27(7):949-957. doi: 10.1016/j.cmi.2021.03.003. Epub 2021 Apr 1. Clin Microbiol Infect. 2021. PMID: 33813117 Free PMC article.
-
Implementation of the trial emulation approach in medical research: a scoping review.BMC Med Res Methodol. 2023 Aug 16;23(1):186. doi: 10.1186/s12874-023-02000-9. BMC Med Res Methodol. 2023. PMID: 37587484 Free PMC article. Review.
-
Beta-Blockers in COPD: A Methodological Review of the Observational Studies.COPD. 2018 Oct;15(5):520-525. doi: 10.1080/15412555.2018.1554637. COPD. 2018. PMID: 30822238 Review.
Cited by
-
Tracing In-Hospital COVID-19 Outcomes: A Multistate Model Exploration (TRACE).Life (Basel). 2024 Sep 21;14(9):1195. doi: 10.3390/life14091195. Life (Basel). 2024. PMID: 39337977 Free PMC article.
-
A Systematic Review of Nirmatrelvir/Ritonavir and Molnupiravir for the Treatment of Coronavirus Disease 2019.Open Forum Infect Dis. 2024 Sep 7;11(9):ofae497. doi: 10.1093/ofid/ofae497. eCollection 2024 Sep. Open Forum Infect Dis. 2024. PMID: 39286035 Free PMC article. Clinical Trial.
References
-
- Cohen JB, D'Agostino McGowan L, Jensen ET, Rigdon J, South AM. Evaluating sources of bias in observational studies of angiotensin-converting enzyme inhibitor/angiotensin II receptor blocker use during COVID-19: beyond confounding. J Hypertens. (2021) 39:795–805. doi: 10.1097/HJH.0000000000002706, PMID: - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

