A retrospective study of dose-dense paclitaxel and carboplatin plus bevacizumab as first-line treatment of advanced epithelial ovarian cancer
- PMID: 38576344
- PMCID: PMC11543256
- DOI: 10.3802/jgo.2024.35.e76
A retrospective study of dose-dense paclitaxel and carboplatin plus bevacizumab as first-line treatment of advanced epithelial ovarian cancer
Abstract
Objective: This study compared the effectiveness, safety, and tolerability of dose-dense paclitaxel and carboplatin plus bevacizumab (ddTC+Bev) with ddTC for advanced ovarian cancer.
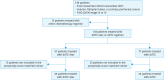
Methods: We retrospectively analyzed the clinical records of 134 patients who received ddTC+Bev or ddTC as first-line chemotherapy for stage III-IV ovarian cancer. Progression-free survival as primary endpoint of this study was compared using the log-rank test. Cox proportional hazards model and propensity score matching (PSM) were used to analyze prognostic factors, and the frequency of adverse events was examined using the χ² test.
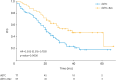
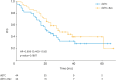
Results: We categorized 134 patients in the ddTC+Bev (n=57) and ddTC (n=77) groups who started treatment at four related institutions from November 2013 to December 2017. No patients used poly (ADP-ribose) polymerase inhibitors as the first line maintenance therapy. The progression-free survival (PFS) of the ddTC+Bev group had a significantly better prognosis than that of the ddTC group (hazard ratio [HR]=0.50; 95% confidence interval [CI]=0.32-0.79; p<0.003). Multivariate analysis showed that ddTC+Bev regimen was a prognostic factor. However, intergroup comparison using PSM revealed that the PFS of the ddTC+Bev group had a nonsignificantly better prognosis than that of the ddTC group (HR=0.70; 95% CI=0.41-1.20; p=0.189). Few adverse events above G3 were noted for ddTC+Bev, which were sufficiently tolerable.
Conclusion: This study could not demonstrate that adding Bev to ddTC improves prognosis. Further studies with more cases are warranted.
Keywords: Angiogenesis; Bevacizumab; Drug Therapy; Ovarian Neoplasms; Paclitaxel.
© 2024. Asian Society of Gynecologic Oncology, Korean Society of Gynecologic Oncology, and Japan Society of Gynecologic Oncology.
Conflict of interest statement
Aikou Okamoto has honoraria from Takeda Pharmaceutical Company Ltd., AstraZeneca K.K., MSD K.K., Mochida Pharmaceutical Co., Ltd., Bayer Holding Ltd., Kaken Pharmaceutical Co., Ltd., ASKA Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Kissei Pharmaceutical Co., Ltd., Fuji Pharma Co., Ltd., Zeria Pharmaceutical Co., Ltd., Eisai Co., Ltd., and Takeda Pharmaceutical Company Ltd. Also, Dr. Okamoto was supported by research funding from Meiji Holdings Co., Ltd., Fuji Pharma Co., Ltd., Taiho Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Kaken Pharmaceutical Co., Ltd., Nippon Shinyaku Co., Ltd., ASKA Pharmaceutical Co., Ltd., Mochida Pharmaceutical Co., Ltd., MSD K.K., Eisai Co., Ltd., Takeda Pharmaceutical Company Ltd., Mochida Pharmaceutical Co., Ltd, Linical Co., Ltd., Pfizer Japan Inc., Gyne Mom Co.Ltd, Terumo Corporation, Kissei Pharmaceutical Co., Ltd., AstraZeneca K.K., Tsumura & Co., and Daiichi Sankyo Co., Ltd.
Figures
Similar articles
-
Therapeutic effect of dose-dense paclitaxel plus carboplatin with or without bevacizumab for Japanese patients with epithelial ovarian cancer.Int J Clin Oncol. 2024 Sep;29(9):1364-1379. doi: 10.1007/s10147-024-02559-3. Epub 2024 Jun 12. Int J Clin Oncol. 2024. PMID: 38865025
-
A randomized phase II/III trial of conventional paclitaxel and carboplatin with or without bevacizumab versus dose-dense paclitaxel and carboplatin with or without bevacizumab, in stage IVB, recurrent, or persistent cervical carcinoma (JCOG1311): Primary analysis.Gynecol Oncol. 2021 Aug;162(2):292-298. doi: 10.1016/j.ygyno.2021.05.007. Epub 2021 May 18. Gynecol Oncol. 2021. PMID: 34016453 Clinical Trial.
-
First-line bevacizumab plus chemotherapy in Chinese patients with stage III/IV epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer: a phase III randomized controlled trial.J Gynecol Oncol. 2024 Sep;35(5):e99. doi: 10.3802/jgo.2024.35.e99. Epub 2024 Apr 22. J Gynecol Oncol. 2024. PMID: 38872480 Free PMC article. Clinical Trial.
-
Bevacizumab combination therapy: a review of its use in patients with epithelial ovarian, fallopian tube, or primary peritoneal cancer.BioDrugs. 2013 Aug;27(4):375-92. doi: 10.1007/s40259-013-0043-4. BioDrugs. 2013. PMID: 23728884 Review.
-
Dose-dense approaches to ovarian cancer treatment.Curr Treat Options Oncol. 2015 May;16(5):21. doi: 10.1007/s11864-015-0338-4. Curr Treat Options Oncol. 2015. PMID: 25859831 Review.
References
-
- du Bois A, Quinn M, Thigpen T, Vermorken J, Avall-Lundqvist E, Bookman M, et al. 2004 consensus statements on the management of ovarian cancer: final document of the 3rd International Gynecologic Cancer Intergroup Ovarian Cancer Consensus Conference (GCIG OCCC 2004) Ann Oncol. 2005;16(Suppl 8):v1117–v1112. - PubMed
-
- Katsumata N, Yasuda M, Isonishi S, Takahashi F, Michimae H, Kimura E, et al. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–1026. - PubMed
-
- Katsumata N, Yasuda M, Takahashi F, Isonishi S, Jobo T, Aoki D, et al. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. - PubMed
-
- Harano K, Terauchi F, Katsumata N, Takahashi F, Yasuda M, Takakura S, et al. Quality-of-life outcomes from a randomized phase III trial of dose-dense weekly paclitaxel and carboplatin compared with conventional paclitaxel and carboplatin as a first-line treatment for stage II-IV ovarian cancer: Japanese Gynecologic Oncology Group Trial (JGOG3016) Ann Oncol. 2014;25:251–257. - PubMed
-
- Clamp AR, James EC, McNeish IA, Dean A, Kim JW, O’Donnell DM, et al. Weekly dose-dense chemotherapy in first-line epithelial ovarian, fallopian tube, or primary peritoneal carcinoma treatment (ICON8): primary progression free survival analysis results from a GCIG phase 3 randomised controlled trial. Lancet. 2019;394:2084–2095. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

