Cx43 hemichannels and panx1 channels contribute to ethanol-induced astrocyte dysfunction and damage
- PMID: 38576018
- PMCID: PMC10996276
- DOI: 10.1186/s40659-024-00493-2
Cx43 hemichannels and panx1 channels contribute to ethanol-induced astrocyte dysfunction and damage
Abstract
Background: Alcohol, a widely abused drug, significantly diminishes life quality, causing chronic diseases and psychiatric issues, with severe health, societal, and economic repercussions. Previously, we demonstrated that non-voluntary alcohol consumption increases the opening of Cx43 hemichannels and Panx1 channels in astrocytes from adolescent rats. However, whether ethanol directly affects astroglial hemichannels and, if so, how this impacts the function and survival of astrocytes remains to be elucidated.
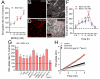
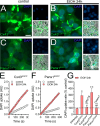
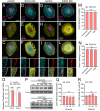
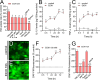
Results: Clinically relevant concentrations of ethanol boost the opening of Cx43 hemichannels and Panx1 channels in mouse cortical astrocytes, resulting in the release of ATP and glutamate. The activation of these large-pore channels is dependent on Toll-like receptor 4, P2X7 receptors, IL-1β and TNF-α signaling, p38 mitogen-activated protein kinase, and inducible nitric oxide (NO) synthase. Notably, the ethanol-induced opening of Cx43 hemichannels and Panx1 channels leads to alterations in cytokine secretion, NO production, gliotransmitter release, and astrocyte reactivity, ultimately impacting survival.
Conclusion: Our study reveals a new mechanism by which ethanol impairs astrocyte function, involving the sequential stimulation of inflammatory pathways that further increase the opening of Cx43 hemichannels and Panx1 channels. We hypothesize that targeting astroglial hemichannels could be a promising pharmacological approach to preserve astrocyte function and synaptic plasticity during the progression of various alcohol use disorders.
Keywords: Alcoholism and neuroinflammation; Astrocyte; Connexin-43; Ethanol; Hemichannels; Pannexin-1.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
HIV gp120 Protein Increases the Function of Connexin 43 Hemichannels and Pannexin-1 Channels in Astrocytes: Repercussions on Astroglial Function.Int J Mol Sci. 2020 Apr 3;21(7):2503. doi: 10.3390/ijms21072503. Int J Mol Sci. 2020. PMID: 32260308 Free PMC article.
-
Heavy Alcohol Exposure Activates Astroglial Hemichannels and Pannexons in the Hippocampus of Adolescent Rats: Effects on Neuroinflammation and Astrocyte Arborization.Front Cell Neurosci. 2018 Dec 4;12:472. doi: 10.3389/fncel.2018.00472. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30564103 Free PMC article.
-
Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes.Glia. 2019 Aug;67(8):1598-1619. doi: 10.1002/glia.23631. Epub 2019 Apr 29. Glia. 2019. PMID: 31033038
-
Connexin and pannexin hemichannels in inflammatory responses of glia and neurons.Brain Res. 2012 Dec 3;1487:3-15. doi: 10.1016/j.brainres.2012.08.042. Epub 2012 Sep 10. Brain Res. 2012. PMID: 22975435 Free PMC article. Review.
-
Glial hemichannels and their involvement in aging and neurodegenerative diseases.Rev Neurosci. 2012 Jan 26;23(2):163-77. doi: 10.1515/revneuro-2011-0065. Rev Neurosci. 2012. PMID: 22499675 Review.
Cited by
-
Acute activation of hemichannels by ethanol leads to Ca2+-dependent gliotransmitter release in astrocytes.Front Cell Dev Biol. 2024 Jun 21;12:1422978. doi: 10.3389/fcell.2024.1422978. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 38974144 Free PMC article.
-
Hemichannels contribute to mitochondrial Ca2+ and morphology alterations evoked by ethanol in astrocytes.Front Cell Dev Biol. 2024 Jul 26;12:1434381. doi: 10.3389/fcell.2024.1434381. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39129788 Free PMC article.
References
-
- Organization WH. Global status report on alcohol and health 2018. Geneva: Licence: CC BY-NC-SA 3.0 IGO.; 2018.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

