Sertaconazole 300 mg versus clotrimazole 500 mg vaginal suppository for treating pregnant women with acute vaginal candidiasis: a double-blinded, randomized trial
- PMID: 38575932
- PMCID: PMC10993551
- DOI: 10.1186/s12884-024-06440-z
Sertaconazole 300 mg versus clotrimazole 500 mg vaginal suppository for treating pregnant women with acute vaginal candidiasis: a double-blinded, randomized trial
Abstract
Background: Vaginal candidiasis (VC) commonly affects pregnant women. Traditionally, clotrimazole vaginal tablets (CLO) have been the cornerstone of management. However, sertaconazole ovules (SER) offer a novel topical antimycotic option. This double-blinded, randomized trial evaluated the efficacy of single-dose SER and CLO in treating acute VC during pregnancy.
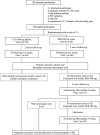
Methods: From June 2020 to May 2021, this trial recruited pregnant women aged ≥ 18 years with VC symptoms (abnormal vaginal discharge and/or vulvar/vaginal itching) confirmed by microscopy. Participants with ≥ 4 VC episodes in the prior year, immunocompromised status, or imidazole contraindications and those who were absent at the 2-week follow-up were excluded. Participants were randomized to receive either 300 mg SER or 500 mg CLO. Evaluations 2 weeks after the initial medication administration included clinical cure (self-reported resolution of all symptoms), microscopic cure (pseudohyphal absence), patient satisfaction, side effects, and time to clinical cure. Participants with persistent VC received weekly SER doses until delivery. Assessments of recurrence and pregnancy outcomes were done.
Results: The analysis included 96 participants (48 per group, mean age 27.4 ± 7.4 years, gestational age at diagnosis 22.9 ± 6.4 weeks). Without statistical significance, SER achieved a higher clinical cure rate (62.5% vs 50%, p = 0.217; a mean difference of 12.5%, 95%CI: -17.5% to 42.5%; and a rate ratio of 1.25, 95%CI: 0.71 to 2.23) and a lower microscopic cure (47.9% vs. 62.5%, p = 0.151; a mean difference of -14.6%, 95%CI: -44.3% to 15.1%; and a rate ratio of 0.77, 95%CI: 0.43 to 1.37). The two groups had comparable times to clinical cure (SER: 3.1 ± 1.8 days, CLO: 3.4 ± 2.7 days; p = 0.848) and substantial satisfaction rates (SER: 66.7%, CLO: 60.4%; p = 0.753). No side effects were reported. Of 60 participants who gave birth at Siriraj Hospital, there were no significant differences in pregnancy outcomes. Repeated SER dosing eradicated symptoms and enhanced the microscopic cure rate. Recurrence was observed in four SER and two CLO participants within 1-2 months.
Conclusion: In the treatment of acute VC during pregnancy, 300 mg SER and 500 mg CLO exhibited comparable efficacy in terms of clinical and microscopic cure rates, satisfaction, side effects, time to clinical cure, recurrence rates, and pregnancy outcomes.
Trial registration: TCTR20190308004 (registration date March 8, 2019).
Keywords: Clotrimazole; Sertaconazole; Pregnancy outcomes; Pregnant women; Single dose; Vaginal candidiasis.
© 2024. The Author(s).
Conflict of interest statement
Bayer, Thailand, and Pacific Health Care, Thailand, donated the study medications and supported some project expenses, including participants’ transportation (totaling 150,000 Baht). C.C. and M.T. received honoraria from these companies for speaking at academic events. However, the companies had no role in the data analysis or manuscript preparation. T.R. and C.K. report no competing interests.
Similar articles
-
[A multicenter, randomized, open and positive parallel controlled clinical study of clotrimazole vaginal expansion suppository and vaginal tablet in the treatment of mild and moderate vulvovaginal candidiasis].Zhonghua Fu Chan Ke Za Zhi. 2020 Oct 25;55(10):697-702. doi: 10.3760/cma.j.cn112141-20200403-00293. Zhonghua Fu Chan Ke Za Zhi. 2020. PMID: 33120482 Clinical Trial. Chinese.
-
Vaginal tablets of dequalinium chloride 10 mg versus clotrimazole 100 mg for vaginal candidiasis: a double-blind, randomized study.Arch Gynecol Obstet. 2021 Jan;303(1):151-160. doi: 10.1007/s00404-020-05784-z. Epub 2020 Sep 17. Arch Gynecol Obstet. 2021. PMID: 32940765 Clinical Trial.
-
Sertaconazole: a review of its use in the management of superficial mycoses in dermatology and gynaecology.Drugs. 2009;69(3):339-59. doi: 10.2165/00003495-200969030-00009. Drugs. 2009. PMID: 19275277 Review.
-
Comparative study of fluconazole and clotrimazole for the treatment of vulvovaginal candidiasis.Sex Transm Dis. 1995 Jul-Aug;22(4):228-30. doi: 10.1097/00007435-199507000-00005. Sex Transm Dis. 1995. PMID: 7482105 Clinical Trial.
-
Oral versus intra-vaginal imidazole and triazole anti-fungal treatment of uncomplicated vulvovaginal candidiasis (thrush).Cochrane Database Syst Rev. 2007 Oct 17;(4):CD002845. doi: 10.1002/14651858.CD002845.pub2. Cochrane Database Syst Rev. 2007. Update in: Cochrane Database Syst Rev. 2020 Aug 24;8:CD002845. doi: 10.1002/14651858.CD002845.pub3. PMID: 17943774 Updated. Review.
References
-
- Chayachinda C, Thamkhantho M, Chalermchockcharoenkit A, Nuengton C, Thipmontree W. Characteristics of clients at the Siriraj Female STD Clinic during 2011–2015. Siriraj Medical Bulletin. 2018;11:182–189.
-
- Verma K, Bhat M, Baniya G. A comparative study of antifungal activity of topical per vaginal application of tablet Sertaconazole and Clotrimazole in cases of vulvovaginal candidiasis. Int J Health Sci Res. 2015;5:111–115.
-
- Roongpisuthipong A, Chalermchockcharoenkit A, Sirimai K, Wanitpongpan P, Jaishuen A, Foongladda S, et al. Safety and efficacy of a new imidazole fungicide, Sertaconazole, in the treatment of fungal vulvo-vaginitis: a comparative study using Fluconazole and Clotrimazole. Asian Biomedicine. 2010;4:443–448. doi: 10.2478/abm-2010-0054. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources