Cloning, functional expression, and pharmacological characterization of inwardly rectifying potassium channels (Kir) from Apis mellifera
- PMID: 38570597
- PMCID: PMC10991380
- DOI: 10.1038/s41598-024-58234-0
Cloning, functional expression, and pharmacological characterization of inwardly rectifying potassium channels (Kir) from Apis mellifera
Abstract
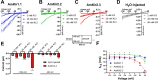
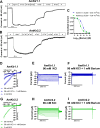
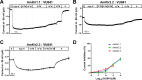
Potassium channels belong to the super family of ion channels and play a fundamental role in cell excitability. Kir channels are potassium channels with an inwardly rectifying property. They play a role in setting the resting membrane potential of many excitable cells including neurons. Although putative Kir channel family genes can be found in the Apis mellifera genome, their functional expression, biophysical properties, and sensitivity to small molecules with insecticidal activity remain to be investigated. We cloned six Kir channel isoforms from Apis mellifera that derive from two Kir genes, AmKir1 and AmKir2, which are present in the Apis mellifera genome. We studied the tissue distribution, the electrophysiological and pharmacological characteristics of three isoforms that expressed functional currents (AmKir1.1, AmKir2.2, and AmKir2.3). AmKir1.1, AmKir2.2, and AmKir2.3 isoforms exhibited distinct characteristics when expressed in Xenopus oocytes. AmKir1.1 exhibited the largest potassium currents and was impermeable to cesium whereas AmKir2.2 and AmKir2.3 exhibited smaller currents but allowed cesium to permeate. AmKir1 exhibited faster opening kinetics than AmKir2. Pharmacological experiments revealed that both AmKir1.1 and AmKir2.2 are blocked by the divalent ion barium, with IC50 values of 10-5 and 10-6 M, respectively. The concentrations of VU041, a small molecule with insecticidal properties required to achieve a 50% current blockade for all three channels were higher than those needed to block Kir channels in other arthropods, such as the aphid Aphis gossypii and the mosquito Aedes aegypti. From this, we conclude that Apis mellifera AmKir channels exhibit lower sensitivity to VU041.
Keywords: Apis mellifera; Cloning; Expression; Insecticides; Insects; Kir channels; Potassium channels; VU041.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Cloning and functional characterization of inward-rectifying potassium (Kir) channels from Malpighian tubules of the mosquito Aedes aegypti.Insect Biochem Mol Biol. 2013 Jan;43(1):75-90. doi: 10.1016/j.ibmb.2012.09.009. Epub 2012 Oct 17. Insect Biochem Mol Biol. 2013. PMID: 23085358 Free PMC article.
-
Inward rectifier potassium (Kir) channels in the soybean aphid Aphis glycines: Functional characterization, pharmacology, and toxicology.J Insect Physiol. 2018 Oct;110:57-65. doi: 10.1016/j.jinsphys.2018.09.001. Epub 2018 Sep 6. J Insect Physiol. 2018. PMID: 30196125 Free PMC article.
-
Biophysical and pharmacological characterization of inwardly rectifying K+ currents in rat spinal cord astrocytes.J Neurophysiol. 1995 Jan;73(1):333-46. doi: 10.1152/jn.1995.73.1.333. J Neurophysiol. 1995. PMID: 7714576
-
Expression, localization, and functional properties of inwardly rectifying K+ channels in the kidney.Am J Physiol Renal Physiol. 2020 Feb 1;318(2):F332-F337. doi: 10.1152/ajprenal.00523.2019. Epub 2019 Dec 16. Am J Physiol Renal Physiol. 2020. PMID: 31841387 Free PMC article. Review.
-
Cholesterol Binding Sites in Inwardly Rectifying Potassium Channels.Adv Exp Med Biol. 2019;1135:119-138. doi: 10.1007/978-3-030-14265-0_7. Adv Exp Med Biol. 2019. PMID: 31098814 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

