Targeted Protein O-GlcNAcylation Using Bifunctional Small Molecules
- PMID: 38561350
- PMCID: PMC11009946
- DOI: 10.1021/jacs.3c14380
Targeted Protein O-GlcNAcylation Using Bifunctional Small Molecules
Abstract
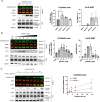
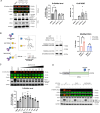
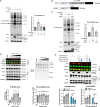
Protein O-linked β-N-acetylglucosamine modification (O-GlcNAcylation) plays a crucial role in regulating essential cellular processes. The disruption of the homeostasis of O-GlcNAcylation has been linked to various human diseases, including cancer, diabetes, and neurodegeneration. However, there are limited chemical tools for protein- and site-specific O-GlcNAc modification, rendering the precise study of the O-GlcNAcylation challenging. To address this, we have developed heterobifunctional small molecules, named O-GlcNAcylation TArgeting Chimeras (OGTACs), which enable protein-specific O-GlcNAcylation in living cells. OGTACs promote O-GlcNAcylation of proteins such as BRD4, CK2α, and EZH2 in cellulo by recruiting FKBP12F36V-fused O-GlcNAc transferase (OGT), with temporal, magnitude, and reversible control. Overall, the OGTACs represent a promising approach for inducing protein-specific O-GlcNAcylation, thus enabling functional dissection and offering new directions for O-GlcNAc-targeting therapeutic development.
Conflict of interest statement
The authors declare the following competing financial interest(s): B.W.M and B.W.-L.N. are inventors on a U.S. non-provisional patent application submitted by The Chinese University of Hong Kong that covers the described bifunctional molecules.
Figures


Similar articles
-
Feedback Regulation of O-GlcNAc Transferase through Translation Control to Maintain Intracellular O-GlcNAc Homeostasis.Int J Mol Sci. 2021 Mar 27;22(7):3463. doi: 10.3390/ijms22073463. Int J Mol Sci. 2021. PMID: 33801653 Free PMC article.
-
O-GlcNAc modification affects the ATM-mediated DNA damage response.Biochim Biophys Acta. 2012 Oct;1820(10):1678-85. doi: 10.1016/j.bbagen.2012.06.013. Epub 2012 Jul 1. Biochim Biophys Acta. 2012. PMID: 22759405
-
O-GlcNAcylation in cancer development and immunotherapy.Cancer Lett. 2023 Jul 10;566:216258. doi: 10.1016/j.canlet.2023.216258. Epub 2023 Jun 4. Cancer Lett. 2023. PMID: 37279852 Review.
-
Changes in O-Linked N-Acetylglucosamine (O-GlcNAc) Homeostasis Activate the p53 Pathway in Ovarian Cancer Cells.J Biol Chem. 2016 Sep 2;291(36):18897-914. doi: 10.1074/jbc.M116.734533. Epub 2016 Jul 11. J Biol Chem. 2016. PMID: 27402830 Free PMC article.
-
[Protein O-GlcNAcylation and regulation of cell signalling: involvement in pathophysiology].Biol Aujourdhui. 2014;208(2):109-17. doi: 10.1051/jbio/2014015. Epub 2014 Sep 8. Biol Aujourdhui. 2014. PMID: 25190571 Review. French.
Cited by
-
O-GlcNAc informatics: advances and trends.Anal Bioanal Chem. 2025 Feb;417(5):895-905. doi: 10.1007/s00216-024-05531-2. Epub 2024 Sep 18. Anal Bioanal Chem. 2025. PMID: 39294469 Review.
-
Current Technologies Unraveling the Significance of Post-Translational Modifications (PTMs) as Crucial Players in Neurodegeneration.Biomolecules. 2024 Jan 16;14(1):118. doi: 10.3390/biom14010118. Biomolecules. 2024. PMID: 38254718 Free PMC article. Review.
-
The non-catalytic domains of O-GlcNAc cycling enzymes present new opportunities for function-specific control.Curr Opin Chem Biol. 2024 Aug;81:102476. doi: 10.1016/j.cbpa.2024.102476. Epub 2024 Jun 10. Curr Opin Chem Biol. 2024. PMID: 38861851 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

