Recent progress in combination therapy of oncolytic vaccinia virus
- PMID: 38558795
- PMCID: PMC10979700
- DOI: 10.3389/fimmu.2024.1272351
Recent progress in combination therapy of oncolytic vaccinia virus
Abstract
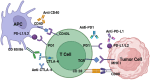
In recent years, oncolytic viruses have emerged as promising agents for treating various cancers. An oncolytic virus is a non-pathogenic virus that, due to genetic manipulation, tends to replicate in and cause lysis of cancerous cells while leaving healthy cells unaffected. Among these viruses, vaccinia virus is an attractive platform for use as an oncolytic platform due to its 190 Kb genome with a high capacity for encoding therapeutic payloads. Combining oncolytic VV therapy with other conventional cancer treatments has been shown to be synergistic and more effective than monotherapies. Additionally, OVV can be used as a vector to deliver therapeutic payloads, alone or in combination with other treatments, to increase overall efficacy. Here, we present a comprehensive analysis of preclinical and clinical studies that have evaluated the efficacy of oncolytic vaccinia viruses in cancer immunotherapy. We discuss the outcomes of these studies, including tumor regression rates, overall survival benefits, and long-term responses. Moreover, we provide insights into the challenges and limitations associated with oncolytic vaccinia virus- based therapies, including immune evasion mechanisms, potential toxicities, and the development of resistance.
Keywords: cancer therapy; combination therapy; immunotherapy; oncolytic vaccinia virus; oncolytic virus.
Copyright © 2024 Mirbahari, Da Silva, Zúñiga, Kooshki Zamani, St-Laurent, Totonchi and Azad.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Preclinical and clinical trials of oncolytic vaccinia virus in cancer immunotherapy: a comprehensive review.Cancer Biol Med. 2023 Aug 23;20(9):646-61. doi: 10.20892/j.issn.2095-3941.2023.0202. Cancer Biol Med. 2023. PMID: 37615308 Free PMC article. Review.
-
Vaccinia virus-mediated cancer immunotherapy: cancer vaccines and oncolytics.J Immunother Cancer. 2019 Jan 9;7(1):6. doi: 10.1186/s40425-018-0495-7. J Immunother Cancer. 2019. PMID: 30626434 Free PMC article. Review.
-
Enhanced antitumor efficacy of a novel oncolytic vaccinia virus encoding a fully monoclonal antibody against T-cell immunoglobulin and ITIM domain (TIGIT).EBioMedicine. 2021 Feb;64:103240. doi: 10.1016/j.ebiom.2021.103240. Epub 2021 Feb 10. EBioMedicine. 2021. PMID: 33581644 Free PMC article.
-
Progress in gene therapy using oncolytic vaccinia virus as vectors.J Cancer Res Clin Oncol. 2018 Dec;144(12):2433-2440. doi: 10.1007/s00432-018-2762-x. Epub 2018 Oct 6. J Cancer Res Clin Oncol. 2018. PMID: 30293118 Review.
-
Engineering oncolytic vaccinia virus with functional peptides through mild and universal strategy.Anal Bioanal Chem. 2019 Feb;411(4):925-933. doi: 10.1007/s00216-018-1519-3. Epub 2018 Dec 7. Anal Bioanal Chem. 2019. PMID: 30523361
Cited by
-
Strategies for engineering oncolytic viruses to enhance cancer immunotherapy.Front Pharmacol. 2024 Sep 6;15:1450203. doi: 10.3389/fphar.2024.1450203. eCollection 2024. Front Pharmacol. 2024. PMID: 39309012 Free PMC article. Review.
References
-
- Andreansky S, Soroceanu L, Flotte ER, Chou J, Markert JM, Gillespie GY, et al. . Evaluation of genetically engineered herpes simplex viruses as oncolytic agents for human Malignant brain tumors. Cancer Res. (1997) 57:1502–9. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

