Reproductive system, temperature, and genetic background effects in experimentally evolving populations of Caenorhabditis elegans
- PMID: 38557670
- PMCID: PMC10984399
- DOI: 10.1371/journal.pone.0300276
Reproductive system, temperature, and genetic background effects in experimentally evolving populations of Caenorhabditis elegans
Abstract
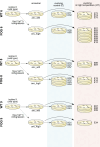
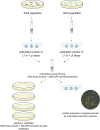
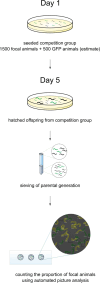
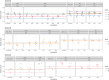
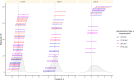
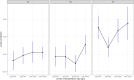
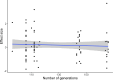
Experimental evolution (EE) is a powerful research framework for gaining insights into many biological questions, including the evolution of reproductive systems. We designed a long-term and highly replicated EE project using the nematode C. elegans, with the main aim of investigating the impact of reproductive system on adaptation and diversification under environmental challenge. From the laboratory-adapted strain N2, we derived isogenic lines and introgressed the fog-2(q71) mutation, which changes the reproductive system from nearly exclusive selfing to obligatory outcrossing, independently into 3 of them. This way, we obtained 3 pairs of isogenic ancestral populations differing in reproductive system; from these, we derived replicate EE populations and let them evolve in either novel (increased temperature) or control conditions for over 100 generations. Subsequently, fitness of both EE and ancestral populations was assayed under the increased temperature conditions. Importantly, each population was assayed in 2-4 independent blocks, allowing us to gain insight into the reproducibility of fitness scores. We expected to find upward fitness divergence, compared to ancestors, in populations which had evolved in this treatment, particularly in the outcrossing ones due to the benefits of genetic shuffling. However, our data did not support these predictions. The first major finding was very strong effect of replicate block on populations' fitness scores. This indicates that despite standardization procedures, some important environmental effects were varying among blocks, and possibly compounded by epigenetic inheritance. Our second key finding was that patterns of EE populations' divergence from ancestors differed among the ancestral isolines, suggesting that research conclusions derived for any particular genetic background should never be generalized without sampling a wider set of backgrounds. Overall, our results support the calls to pay more attention to biological variability when designing studies and interpreting their results, and to avoid over-generalizations of outcomes obtained for specific genetic and/or environmental conditions.
Copyright: © 2024 Baran et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Evolution of fertilization ability in obligatorily outcrossing populations of Caenorhabditis elegans.PeerJ. 2023 Sep 8;11:e15825. doi: 10.7717/peerj.15825. eCollection 2023. PeerJ. 2023. PMID: 37701823 Free PMC article.
-
Mitonuclear Mismatch is Associated With Increased Male Frequency, Outcrossing, and Male Sperm Size in Experimentally-Evolved C. elegans.Front Genet. 2022 Mar 11;13:742272. doi: 10.3389/fgene.2022.742272. eCollection 2022. Front Genet. 2022. PMID: 35360860 Free PMC article.
-
Variation in rates of spontaneous male production within the nematode species Pristionchus pacificus supports an adaptive role for males and outcrossing.BMC Evol Biol. 2017 Feb 23;17(1):57. doi: 10.1186/s12862-017-0873-7. BMC Evol Biol. 2017. PMID: 28228092 Free PMC article.
-
[Genetics and evolution of developmental plasticity in the nematode C. elegans: Environmental induction of the dauer stage].Biol Aujourdhui. 2020;214(1-2):45-53. doi: 10.1051/jbio/2020006. Epub 2020 Aug 10. Biol Aujourdhui. 2020. PMID: 32773029 Review. French.
-
Biology is the root of variability: cautionary tales in Caenorhabditis elegans biology.Biochem Soc Trans. 2019 Jun 28;47(3):887-896. doi: 10.1042/BST20190001. Epub 2019 May 24. Biochem Soc Trans. 2019. PMID: 31127069 Review.
References
-
- Carroll SB. Morphological Complexity and Diversity. Nature. 2001; 409(6823), 1102–1109. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

