Biomaterial-mediated intracellular control of macrophages for cell therapy in pro-inflammatory and pro-fibrotic conditions
- PMID: 38547831
- PMCID: PMC11264195
- DOI: 10.1016/j.biomaterials.2024.122545
Biomaterial-mediated intracellular control of macrophages for cell therapy in pro-inflammatory and pro-fibrotic conditions
Abstract
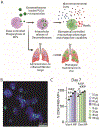
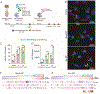
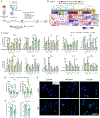
Macrophages are key modulators of all inflammatory diseases and essential for their resolution, making macrophage cell therapy a promising strategy for regenerative medicine. However, since macrophages change rapidly in response to microenvironmental cues, their phenotype must be controlled post-administration. We present a tunable biomaterial-based strategy to control macrophages intracellularly via small molecule-releasing microparticles. Poly(lactic-co-glycolic acid) microparticles encapsulating the anti-inflammatory and anti-fibrotic drug dexamethasone were administered to macrophages in vitro, with uptake rates controlled by different loading regimes. Microparticle dose and dexamethasone content directly affected macrophage phenotype and phagocytic capacity, independent of particle content per cell, leading to an overall pro-reparative, anti-inflammatory, anti-fibrotic phenotype with increased phagocytic and ECM degrading functionality. Intracellularly controlled macrophages partially maintained this phenotype in vivo in a murine pulmonary fibrosis model, with more prominent effects in a pro-fibrotic environment compared to pro-inflammatory. These results suggest that intracellular control using biomaterials has the potential to control macrophage phenotype post-administration, which is essential for successful macrophage cell therapy.
Keywords: Cell therapy; Immunomodulation; Macrophage polarization; Pulmonary fibrosis.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Kara L. Spiller reports financial support was provided by National Heart Lung and Blood Institute. Kara L. Spiller reports financial support was provided by Coulter-Drexel Translational Research Partnership. Kara L. Spiller has patent pending to Drexel University. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Biomaterial-mediated reprogramming of monocytes via microparticle phagocytosis for sustained modulation of macrophage phenotype.Acta Biomater. 2020 Jan 1;101:237-248. doi: 10.1016/j.actbio.2019.11.021. Epub 2019 Nov 13. Acta Biomater. 2020. PMID: 31731024 Free PMC article.
-
Aspirin-Triggered Resolvin D1-modified materials promote the accumulation of pro-regenerative immune cell subsets and enhance vascular remodeling.Acta Biomater. 2017 Apr 15;53:109-122. doi: 10.1016/j.actbio.2017.02.020. Epub 2017 Feb 16. Acta Biomater. 2017. PMID: 28213094 Free PMC article.
-
Modulation of macrophage phenotype via phagocytosis of drug-loaded microparticles.J Biomed Mater Res A. 2019 Jun;107(6):1213-1224. doi: 10.1002/jbm.a.36617. Epub 2019 Feb 11. J Biomed Mater Res A. 2019. PMID: 30672109 Free PMC article.
-
Macrophage phenotypes in tissue repair and the foreign body response: Implications for biomaterial-based regenerative medicine strategies.Acta Biomater. 2021 Oct 1;133:4-16. doi: 10.1016/j.actbio.2021.03.038. Epub 2021 Mar 26. Acta Biomater. 2021. PMID: 33775905 Free PMC article. Review.
-
Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis.Adv Healthc Mater. 2019 Feb;8(4):e1801451. doi: 10.1002/adhm.201801451. Epub 2019 Jan 18. Adv Healthc Mater. 2019. PMID: 30658015 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

