Sex differences in inflammation correlated with estrogen and estrogen receptor-β levels in azoxymethane/dextran sodium sulfate-induced colitis-associated colorectal cancer mice
- PMID: 38545214
- PMCID: PMC10966699
- DOI: 10.1016/j.heliyon.2024.e28121
Sex differences in inflammation correlated with estrogen and estrogen receptor-β levels in azoxymethane/dextran sodium sulfate-induced colitis-associated colorectal cancer mice
Abstract
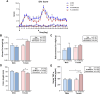
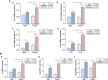
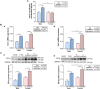
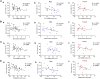
Colorectal cancer (CRC) is a type of cancer that develops in the colon or rectum and is the second leading cause of cancer-related death worldwide. Several epidemiology studies have identified a significant sexual dimorphism in CRC, with women exhibiting a lower incidence rate and delayed onset compared to men. This study aims to investigate the sexual dimorphism in the inflammatory response in colitis-associated CRC and its relationship with estrogen and estrogen receptors. An azoxymethane (AOM)/dextran sodium sulfate (DSS) mouse model was used to induce colitis-associated CRC. Five-week-old male and female mice were randomly assigned into either the control group or the AOM/DSS CRC group, with 10 mice in each group. Colitis-associated CRC was induced by injecting AOM (10 mg/kg) and administering two-cycles of DSS treatment in the drinking water. The results revealed a significant decrease in colon length exclusively in the female group, indicating more severe colonic inflammation (P < 0.01). A significant interaction was identified between sex and AOM/DSS treatment in the female AOM/DSS group, with higher visceral fat weight compared to their male counterparts (P < 0.05). The female AOM/DSS group also exhibited elevated production of M1 macrophage-related pro-inflammatory cytokines, suggesting increased tumor-associated macrophage activity. Surprisingly, the male AOM/DSS group showed a marked increase in serum estradiol levels, while the female AOM/DSS group exhibited a decrease compared to the normal control group. Additionally, a notable upregulation of both estrogen receptor α and estrogen receptor β expression was observed in the colon tissues of the AOM/DSS groups compared to the normal control groups, with estrogen receptor β expression being particularly pronounced in females. Taken together, our findings suggest that a decline in endogenous estrogen and increased estrogen receptors potentially contribute to the pro-inflammatory response in early CRC by augmenting cytokine expressions associated with M1 macrophage polarization in females.
Keywords: Colitis; Colorectal cancer; Estrogen; Estrogen receptor; Macrophage; Sexual dimorphism.
© 2024 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
Effects of 17β-estradiol on colorectal cancer development after azoxymethane/dextran sulfate sodium treatment of ovariectomized mice.Biochem Pharmacol. 2019 Jun;164:139-151. doi: 10.1016/j.bcp.2019.04.011. Epub 2019 Apr 11. Biochem Pharmacol. 2019. PMID: 30981879
-
Effect of Estradiol in an Azoxymethane/Dextran Sulfate Sodium-Treated Mouse Model of Colorectal Cancer: Implication for Sex Difference in Colorectal Cancer Development.Cancer Res Treat. 2019 Apr;51(2):632-648. doi: 10.4143/crt.2018.060. Epub 2018 Aug 1. Cancer Res Treat. 2019. PMID: 30064198 Free PMC article.
-
The germ-free mice monocolonization with Bacteroides fragilis improves azoxymethane/dextran sulfate sodium induced colitis-associated colorectal cancer.Immunopharmacol Immunotoxicol. 2019 Apr;41(2):207-213. doi: 10.1080/08923973.2019.1569047. Epub 2019 Feb 1. Immunopharmacol Immunotoxicol. 2019. PMID: 30706742
-
Murine models of colorectal cancer: the azoxymethane (AOM)/dextran sulfate sodium (DSS) model of colitis-associated cancer.PeerJ. 2023 Oct 31;11:e16159. doi: 10.7717/peerj.16159. eCollection 2023. PeerJ. 2023. PMID: 37927787 Free PMC article. Review.
-
Sexual Dimorphism in Colon Cancer.Front Oncol. 2020 Dec 9;10:607909. doi: 10.3389/fonc.2020.607909. eCollection 2020. Front Oncol. 2020. PMID: 33363037 Free PMC article. Review.
Cited by
-
Development and Validation of Machine Learning Algorithms for Prediction of Colorectal Polyps Based on Electronic Health Records.Biomedicines. 2024 Aug 27;12(9):1955. doi: 10.3390/biomedicines12091955. Biomedicines. 2024. PMID: 39335469 Free PMC article.
-
Role of sex steroids in colorectal cancer: pathomechanisms and medical applications.Am J Cancer Res. 2024 Jul 15;14(7):3200-3221. doi: 10.62347/OEBS6893. eCollection 2024. Am J Cancer Res. 2024. PMID: 39113870 Free PMC article. Review.
References
-
- Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F., Global Cancer Statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA. Cancer J. Clin. 2021;71(3):209–249. - PubMed
-
- Ng S.C., Shi H.Y., Hamidi N., Underwood F.E., Tang W., Benchimol E.I., Panaccione R., Ghosh S., Wu J.C.Y., Chan F.K.L., Sung J.J.Y., Kaplan G.G. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390(10114):2769–2778. - PubMed
-
- McCashland T.M., Brand R., Lyden E., de Garmo P. Gender differences in colorectal polyps and tumors. Am. J. Gastroenterol. 2001;96(3):882–886. - PubMed
-
- Jess T., Rungoe C., Peyrin–Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012;10(6):639–645. - PubMed
LinkOut - more resources
Full Text Sources

