Long-Term Observation of SARS-CoV-2 Vaccination Response upon High Efficacy Treatment in Multiple Sclerosis-A Real-World Scenario
- PMID: 38543930
- PMCID: PMC10974098
- DOI: 10.3390/vaccines12030296
Long-Term Observation of SARS-CoV-2 Vaccination Response upon High Efficacy Treatment in Multiple Sclerosis-A Real-World Scenario
Abstract
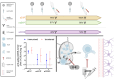
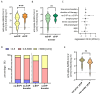
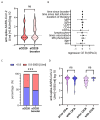
Immunomodulatory and immunosuppressive therapy is needed in people with a chronic neuroinflammatory disease of the central nervous system such as multiple sclerosis (MS). Therefore, MS requires monitoring for and preventing against infectious diseases like SARS-CoV-2. Vaccination and anti-viral treatments are, in particular, recommended for elderly people and people at risk of a severe course of infection and of MS. Here, we asked whether repetitive infection or vaccination influenced responses upon receiving high efficacy treatments, namely sphingosine-1-phosphate receptor modulator (S1P) or anti-CD20 B cell antibody (anti-CD20) treatments. We performed a prospective real-world study of people with MS (pwMS) under S1P or anti-CD20 with repetitive exposure to the SARS-CoV-2 virus or vaccine. The measurement of anti-SARS-CoV-2 antibody titres was performed by two independent immunoassays after initial immunisation and after booster vaccination or infection. Other laboratory and clinical parameters were included in the analysis of influencing factors. As secondary outcomes, lymphocyte and immunoglobulin levels were observed longitudinally under intravenous and subcutaneous anti-CD20 treatment. In a long-term real-world cohort of 201 pwMS, we found that despite lymphopenia upon S1P drugs, the SARS-CoV-2 immunisation response increased both in selective and non-selective S1P (100% and 88% seroconversion, respectively), whereas those under anti-CD20 therapies merely exhibited a slight long-term increase in antibody titres (52% seroconversion). The latter was independent of immunoglobulin or total lymphocyte levels, which mostly remained stable. If the individual was immunised prior to therapy initiation, their levels of SARS-CoV-2 antibodies remained high under treatment. PwMS under non-selective S1P benefit from repetitive vaccination. The risk of an insufficient vaccination response mirrored by lower SARS-CoV-2 antibodies remains in pwMS receiving anti-CD20 treatment, even after repetitive exposure to the vaccine or virus. Due to the compromised vaccination response in CD20-depleting drugs, prompt antiviral treatment might be necessary.
Keywords: SARS-CoV-2; anti-CD20; booster vaccination; efficiency; multiple sclerosis; sphingosine-1-phopshate receptor modulator; vaccination.
Conflict of interest statement
The authors declare no relevant conflicts of interest.
Figures
Similar articles
-
Predictors for insufficient SARS-CoV-2 vaccination response upon treatment in multiple sclerosis.EBioMedicine. 2023 Jan;87:104411. doi: 10.1016/j.ebiom.2022.104411. Epub 2022 Dec 17. EBioMedicine. 2023. PMID: 36535106 Free PMC article.
-
Longitudinal adaptive immune responses following sequential SARS-CoV-2 vaccinations in MS patients on anti-CD20 therapies and sphingosine-1-phosphate receptor modulators.Mult Scler Relat Disord. 2023 Feb;70:104484. doi: 10.1016/j.msard.2022.104484. Epub 2022 Dec 28. Mult Scler Relat Disord. 2023. PMID: 36608538 Free PMC article.
-
Recall response to COVID-19 antigen is preserved in people with multiple sclerosis on anti-CD20 medications - A pilot study.Mult Scler Relat Disord. 2022 Mar;59:103560. doi: 10.1016/j.msard.2022.103560. Epub 2022 Jan 24. Mult Scler Relat Disord. 2022. PMID: 35093840 Free PMC article.
-
Immune responses to SARS-CoV-2 vaccination in multiple sclerosis: a systematic review/meta-analysis.Ann Clin Transl Neurol. 2022 Aug;9(8):1321-1331. doi: 10.1002/acn3.51628. Epub 2022 Jul 19. Ann Clin Transl Neurol. 2022. PMID: 35852423 Free PMC article. Review.
-
Multiple Sclerosis Treatment in the COVID-19 Era: A Risk-Benefit Approach.Neurol Int. 2022 Apr 15;14(2):368-377. doi: 10.3390/neurolint14020030. Neurol Int. 2022. PMID: 35466211 Free PMC article. Review.
References
-
- Hahn M., Groschel S., Groschel K., Uphaus T. Association of Delirium Incidence with Visitation Restrictions due to COVID-19 Pandemic in Patients with Acute Cerebrovascular Disease in a Stroke-Unit Setting: A Retrospective Cohort Study. Gerontology. 2023;69:273–281. doi: 10.1159/000526165. - DOI - PubMed
-
- Sormani M.P., Schiavetti I., Carmisciano L., Cordioli C., Filippi M., Radaelli M., Immovilli P., Capobianco M., De Rossi N., Brichetto G., et al. COVID-19 Severity in Multiple Sclerosis: Putting Data Into Context. Neurol. Neuroimmunol. Neuroinflamm. 2022;9:e1105. doi: 10.1212/NXI.0000000000001105. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

