TRPV4 Activation during Guinea Pig Airway Smooth Muscle Contraction Promotes Ca2+ and Na+ Influx
- PMID: 38543079
- PMCID: PMC10976253
- DOI: 10.3390/ph17030293
TRPV4 Activation during Guinea Pig Airway Smooth Muscle Contraction Promotes Ca2+ and Na+ Influx
Abstract
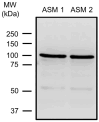
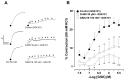
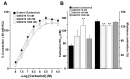
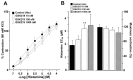
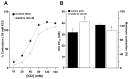
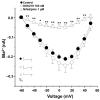
Airway smooth muscle (ASM) contraction is determined by the increase in intracellular Ca2+ concentration ([Ca2+]i) caused by its release from the sarcoplasmic reticulum (SR) or by extracellular Ca2+ influx. Major channels involved in Ca2+ influx in ASM cells are L-type voltage-dependent Ca2+ channels (L-VDCCs) and nonselective cation channels (NSCCs). Transient receptor potential vanilloid 4 (TRPV4) is an NSCC recently studied in ASM. Mechanical stimuli, such as contraction, can activate TRPV4. We investigated the possible activation of TRPV4 by histamine (His)- or carbachol (CCh)-induced contraction in guinea pig ASM. In single myocytes, the TRPV4 agonist (GSK101) evoked an increase in [Ca2+]i, characterized by a slow onset and a plateau phase. The TRPV4 antagonist (GSK219) decreased channel activity by 94%, whereas the Ca2+-free medium abolished the Ca2+ response induced by GSK101. Moreover, GSK101 caused Na+ influx in tracheal myocytes. GSK219 reduced the Ca2+ peak and the Ca2+ plateau triggered by His or CCh. TRPV4 blockade shifted the concentration-response curve relating to His and CCh to the right in tracheal rings and reduced the maximal contraction. Finally, the activation of TRPV4 in single myocytes increased the Ca2+ refilling of the SR. We conclude that contraction of ASM cells after stimulation with His or CCh promotes TRPV4 activation, the subsequent influx of Ca2+ and Na+, and the opening of L-VDCCs. The entry of Ca2+ into ASM cells via TRPV4 and L-VDCCs contributes to optimal smooth muscle contraction.
Keywords: TRPV4; airway smooth muscle; asthma; intracellular Ca2+ concentration; smooth muscle contraction.
Conflict of interest statement
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. Figure 12 of this manuscript was created with BioRender.com.
Figures





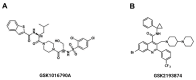
Similar articles
-
TRPV4 Complexes With the Na+/Ca2+ Exchanger and IP3 Receptor 1 to Regulate Local Intracellular Calcium and Tracheal Tension in Mice.Front Physiol. 2019 Dec 6;10:1471. doi: 10.3389/fphys.2019.01471. eCollection 2019. Front Physiol. 2019. PMID: 31866874 Free PMC article.
-
Novel airway smooth muscle-mast cell interactions and a role for the TRPV4-ATP axis in non-atopic asthma.Eur Respir J. 2020 Jul 2;56(1):1901458. doi: 10.1183/13993003.01458-2019. Print 2020 Jul. Eur Respir J. 2020. PMID: 32299856 Free PMC article.
-
Testosterone-induced relaxation involves L-type and store-operated Ca2+ channels blockade, and PGE 2 in guinea pig airway smooth muscle.Pflugers Arch. 2015 Apr;467(4):767-77. doi: 10.1007/s00424-014-1534-y. Epub 2014 May 29. Pflugers Arch. 2015. PMID: 24872164
-
Nonselective cation channels activated by the stimulation of muscarinic receptors in mammalian gastric smooth muscle.J Smooth Muscle Res. 2003 Dec;39(6):231-47. doi: 10.1540/jsmr.39.231. J Smooth Muscle Res. 2003. PMID: 15048016 Review.
-
Mitochondria Structure and Position in the Local Control of Calcium Signals in Smooth Muscle Cells.In: Trebak M, Earley S, editors. Signal Transduction and Smooth Muscle. Oxon (UK): CRC Press/Taylor & Francis; 2018 Aug 6. Chapter 9. In: Trebak M, Earley S, editors. Signal Transduction and Smooth Muscle. Oxon (UK): CRC Press/Taylor & Francis; 2018 Aug 6. Chapter 9. PMID: 31436944 Free Books & Documents. Review.
References
-
- Shetty S.M., Archana S. In: Anatomy and Physiology of the Airway Applied Aspects. Ubaradka R.S., Gupta N., Bidkar P.U., Tripathy D.K., Gupta A., editors. Springer; Singapore: 2023. pp. 21–43. The Airway Manual. - DOI
-
- Montaño L.M., Flores-Soto E., Reyes-García J., Solis-Chagoyán H., Sommer B. Airway smooth muscle functioning in basal, agonists stimulated conditions and novel androgen asthma therapy. Adv. Med. Biol. 2020;157:1–64.
Grants and funding
- IN200522/Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México
- IN220219/Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México
- IA201322/Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México
- IA203924/Dirección General de Asuntos del Personal Académico (DGAPA), Universidad Nacional Autónoma de México
- CF72019-137725/Consejo Nacional de Ciencia y Tecnología, México
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

