Hitting the Target! Challenges and Opportunities for TGF-β Inhibition for the Treatment of Cardiac fibrosis
- PMID: 38543053
- PMCID: PMC10975989
- DOI: 10.3390/ph17030267
Hitting the Target! Challenges and Opportunities for TGF-β Inhibition for the Treatment of Cardiac fibrosis
Abstract
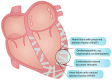
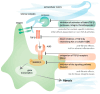
Developing effective anti-fibrotic therapies for heart diseases holds the potential to address unmet needs in several cardiac conditions, including heart failure with preserved ejection fraction, hypertrophic cardiomyopathy, and cardiotoxicity induced by cancer therapy. The inhibition of the primary fibrotic regulator, transforming growth factor (TGF) β, represents an efficient strategy for mitigating fibrosis in preclinical models. However, translating these findings into clinical benefits faces challenges due to potential adverse effects stemming from TGF-β's physiological actions in inflammation and tissue homeostasis. Various strategies exist for inhibiting TGF-β, each associated with a distinct risk of adverse effects. Targeting TGF-β directly or through its signaling pathway proves efficient in reducing fibrosis. However, direct TGF-β blockade may lead to uncontrolled inflammation, especially following myocardial infarction, while interference with the signaling pathway may compromise structural integrity, resulting in issues like insufficient wound healing or ventricular dilatation. Influencing TGF-β activity through interacting signaling pathways, for instance by inhibitors of the renin-angiotensin-aldosterone-system, is insufficiently potent in reducing fibrosis. Targeting activators of latent TGF-β, including ADAMTS enzymes, thrombospondin, and integrins, emerges as a potentially safer strategy to reduce TGF-β-induced fibrosis but it requires the identification of appropriate targets. Encouragement is drawn from promising agents developed for fibrosis in other organs, fueling hope for similar breakthroughs in treating cardiac fibrosis. Such advances depend on overcoming obstacles for the implementation of anti-fibrotic strategies in patients with heart disease, including fibrosis quantification. In this review, insights garnered from interventional and mechanistic studies, obtained through a non-systemic search spanning preclinical and clinical evidence, are summarized to pinpoint the most promising targets for further exploration and development.
Keywords: TGF-β; anti-fibrotic therapy; cardiac fibrosis; heart failure.
Conflict of interest statement
Vistnes is among the inventors of a patent filed by the University of Oslo covering the use of ADAMTS4 inhibition in cardiac remodeling and heart failure (WO2015004209A1). A license agreement with the pharmaceutical company Paradigm Biopharma for the development of pentosane polysulfate in the treatment of cardiac remodeling and heart failure was signed between the University of Oslo and Paradigm in 2017, and Vistnes has performed consulting services for Paradigm to support this development and was among the inventors of a patent covering the use of pentosane polysulfate in heart failure filed by Paradigm Biopharma (AU2022051301). Vistnes has received fees from Novartis, Pharmacosmos, and AstraZeneca (advisory boards), and is the founder of a startup company planning the development of preclinical drug candidates for treating cardiac fibrosis.
Figures
Similar articles
-
Simvastatin Attenuates Cardiac Fibrosis under Pathophysiological Conditions of Heart Failure with Preserved Left Ventricular Ejection Fraction by Inhibiting TGF-β Signaling.Pharmacology. 2024;109(1):43-51. doi: 10.1159/000534933. Epub 2023 Nov 28. Pharmacology. 2024. PMID: 38016432
-
Therapeutic Targets for the Treatment of Cardiac Fibrosis and Cancer: Focusing on TGF-β Signaling.Front Cardiovasc Med. 2020 Mar 10;7:34. doi: 10.3389/fcvm.2020.00034. eCollection 2020. Front Cardiovasc Med. 2020. PMID: 32211422 Free PMC article. Review.
-
MicroRNA-101a suppresses fibrotic programming in isolated cardiac fibroblasts and in vivo fibrosis following trans-aortic constriction.J Mol Cell Cardiol. 2018 Aug;121:266-276. doi: 10.1016/j.yjmcc.2018.07.251. Epub 2018 Jul 24. J Mol Cell Cardiol. 2018. PMID: 30053527
-
Transforming growth factor (TGF)-β signaling in cardiac remodeling.J Mol Cell Cardiol. 2011 Oct;51(4):600-6. doi: 10.1016/j.yjmcc.2010.10.033. Epub 2010 Nov 6. J Mol Cell Cardiol. 2011. PMID: 21059352 Free PMC article. Review.
-
Inhibition of the extracellular enzyme A disintegrin and metalloprotease with thrombospondin motif 4 prevents cardiac fibrosis and dysfunction.Cardiovasc Res. 2023 Aug 19;119(10):1915-1927. doi: 10.1093/cvr/cvad078. Cardiovasc Res. 2023. PMID: 37216909 Free PMC article.
Cited by
-
Mechanisms of Bleomycin-induced Lung Fibrosis: A Review of Therapeutic Targets and Approaches.Cell Biochem Biophys. 2024 Sep;82(3):1845-1870. doi: 10.1007/s12013-024-01384-9. Epub 2024 Jul 2. Cell Biochem Biophys. 2024. PMID: 38955925 Review.
-
Advances and Challenges in Targeting TGF-β Isoforms for Therapeutic Intervention of Cancer: A Mechanism-Based Perspective.Pharmaceuticals (Basel). 2024 Apr 20;17(4):533. doi: 10.3390/ph17040533. Pharmaceuticals (Basel). 2024. PMID: 38675493 Free PMC article. Review.
References
-
- Kato S., Saito N., Kirigaya H., Gyotoku D., Iinuma N., Kusakawa Y., Iguchi K., Nakachi T., Fukui K., Futaki M., et al. Prognostic significance of quantitative assessment of focal myocardial fibrosis in patients with heart failure with preserved ejection fraction. Int. J. Cardiol. 2015;191:314–319. doi: 10.1016/j.ijcard.2015.05.048. - DOI - PubMed
-
- Gulati A., Jabbour A., Ismail T.F., Guha K., Khwaja J., Raza S., Morarji K., Brown T.D.H., Ismail N.A., Dweck M.R., et al. Association of Fibrosis With Mortality and Sudden Cardiac Death in Patients With Nonischemic Dilated Cardiomyopathy. J. Am. Med. Assoc. 2013;309:896–908. doi: 10.1001/jama.2013.1363. - DOI - PubMed
-
- Moreo A., Ambrosio G., De Chiara B., Pu M., Tran T., Mauri F., Raman S.V. Influence of myocardial fibrosis on left ventricular diastolic function: Noninvasive assessment by cardiac magnetic resonance and echo. Circ. Cardiovasc. Imaging. 2009;2:437–443. doi: 10.1161/CIRCIMAGING.108.838367. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

