Fluorimetric Detection of Insulin Misfolding by Probes Derived from Functionalized Fluorene Frameworks
- PMID: 38542833
- PMCID: PMC10975426
- DOI: 10.3390/molecules29061196
Fluorimetric Detection of Insulin Misfolding by Probes Derived from Functionalized Fluorene Frameworks
Abstract
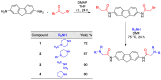
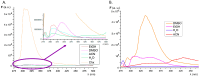
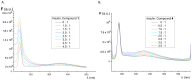
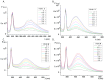
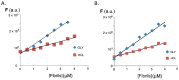
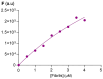
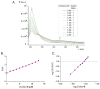
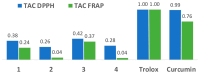
A group of functionalized fluorene derivatives that are structurally similar to the cellular prion protein ligand N,N'-(methylenedi-4,1-phenylene)bis [2-(1-pyrrolidinyl)acetamide] (GN8) have been synthesized. These compounds show remarkable native fluorescence due to the fluorene ring. The substituents introduced at positions 2 and 7 of the fluorene moiety are sufficiently flexible to accommodate the beta-conformational folding that develops in amyloidogenic proteins. Changes in the native fluorescence of these fluorene derivatives provide evidence of transformations in the amyloidogenic aggregation processes of insulin. The increase observed in the fluorescence intensity of the sensors in the presence of native insulin or amyloid aggregates suggest their potential use as fluorescence probes for detecting abnormal conformations; therefore, the compounds can be proposed for use as "turn-on" fluorescence sensors. Protein-sensor dissociation constants are in the 5-10 μM range and an intermolecular charge transfer process between the protein and the sensors can be successfully exploited for the sensitive detection of abnormal insulin conformations. The values obtained for the Stern-Volmer quenching constant for compound 4 as a consequence of the sensor-protein interaction are comparable to those obtained for the reference compound GN8. Fluorene derivatives showed good performance in scavenging reactive oxygen species (ROS), and they show antioxidant capacity according to the FRAP and DPPH assays.
Keywords: antioxidants; fluorescence probes; insulin aggregates detection; insulin amyloid conformation; turn-on fluorescence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
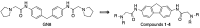
Similar articles
-
Quenching of fluorene fluorescence by single-walled carbon nanotube dispersions with surfactants: application for fluorene quantification in wastewater.Anal Bioanal Chem. 2015 Jun;407(16):4671-82. doi: 10.1007/s00216-015-8669-3. Epub 2015 Apr 19. Anal Bioanal Chem. 2015. PMID: 25893803
-
Spectroscopic properties, excitation, and electron transfer in an anionic water-soluble poly(fluorene-alt-phenylene)-perylenediimide copolymer.J Phys Chem B. 2012 Jun 28;116(25):7548-59. doi: 10.1021/jp3000703. Epub 2012 Jun 18. J Phys Chem B. 2012. PMID: 22554070
-
Singlet-singlet energy transfer in self-assembled systems of the cationic poly{9,9-bis[6-N,N,N-trimethylammonium)hexyl]fluorene-co-1,4-phenylene} with oppositely charged porphyrins.J Phys Chem B. 2009 Dec 17;113(50):16093-100. doi: 10.1021/jp904959h. J Phys Chem B. 2009. PMID: 19925000
-
Structural mechanisms of oligomer and amyloid fibril formation by the prion protein.Chem Commun (Camb). 2018 Jun 14;54(49):6230-6242. doi: 10.1039/c8cc03053g. Chem Commun (Camb). 2018. PMID: 29789820 Review.
-
Interrogating Amyloid Aggregates using Fluorescent Probes.Chem Rev. 2019 Dec 11;119(23):11819-11856. doi: 10.1021/acs.chemrev.9b00404. Epub 2019 Nov 1. Chem Rev. 2019. PMID: 31675223 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

