Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies
- PMID: 38540242
- PMCID: PMC10967866
- DOI: 10.3390/biomedicines12030626
Co-Culture Models: Key Players in In Vitro Neurotoxicity, Neurodegeneration and BBB Modeling Studies
Abstract
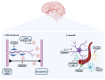
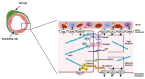
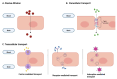
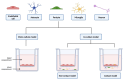
The biological barriers existing in the human body separate the blood circulation from the interstitial fluid in tissues. The blood-brain barrier (BBB) isolates the central nervous system from the bloodstream, presenting a dual role: the protection of the human brain against potentially toxic/harmful substances coming from the blood, while providing nutrients to the brain and removing metabolites. In terms of architectural features, the presence of junctional proteins (that restrict the paracellular transport) and the existence of efflux transporters at the BBB are the two major in vivo characteristics that increase the difficulty in creating an ideal in vitro model for drug permeability studies and neurotoxicity assessments. The purpose of this work is to provide an up-to-date literature review on the current in vitro models used for BBB studies, focusing on the characteristics, advantages, and disadvantages of both primary cultures and immortalized cell lines. An accurate analysis of the more recent and emerging techniques implemented to optimize the in vitro models is also provided, based on the need of recreating as closely as possible the BBB microenvironment. In fact, the acceptance that the BBB phenotype is much more than endothelial cells in a monolayer has led to the shift from single-cell to multicellular models. Thus, in vitro co-culture models have narrowed the gap between recreating as faithfully as possible the human BBB phenotype. This is relevant for permeability and neurotoxicity assays, and for studies related to neurodegenerative diseases. Several studies with these purposes will be also presented and discussed.
Keywords: blood–brain barrier; co-culture models; in vitro; neurodegeneration; neurotoxicity; neurovascular unit.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
In vitro models of the blood-brain barrier.Acta Neurobiol Exp (Wars). 2011;71(1):113-28. doi: 10.55782/ane-2011-1828. Acta Neurobiol Exp (Wars). 2011. PMID: 21499332 Review.
-
Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood-brain barrier model for drug permeability studies.Fluids Barriers CNS. 2013 Nov 22;10(1):33. doi: 10.1186/2045-8118-10-33. Fluids Barriers CNS. 2013. PMID: 24262108 Free PMC article.
-
Comparison of a Rat Primary Cell-Based Blood-Brain Barrier Model With Epithelial and Brain Endothelial Cell Lines: Gene Expression and Drug Transport.Front Mol Neurosci. 2018 May 22;11:166. doi: 10.3389/fnmol.2018.00166. eCollection 2018. Front Mol Neurosci. 2018. PMID: 29872378 Free PMC article.
-
Development of Human in vitro Brain-blood Barrier Model from Induced Pluripotent Stem Cell-derived Endothelial Cells to Predict the in vivo Permeability of Drugs.Neurosci Bull. 2019 Dec;35(6):996-1010. doi: 10.1007/s12264-019-00384-7. Epub 2019 May 11. Neurosci Bull. 2019. PMID: 31079318 Free PMC article.
-
Patented in vitro blood-brain barrier models in CNS drug discovery.Recent Pat CNS Drug Discov. 2011 May 1;6(2):107-18. doi: 10.2174/157488911795933910. Recent Pat CNS Drug Discov. 2011. PMID: 21585327 Review.
Cited by
-
An Overview of Multiple Sclerosis In Vitro Models.Int J Mol Sci. 2024 Jul 16;25(14):7759. doi: 10.3390/ijms25147759. Int J Mol Sci. 2024. PMID: 39063001 Free PMC article. Review.
References
-
- Barichello T., Collodel A., Hasbun R., Morales R. An Overview of the Blood-Brain Barrier. Volume 142. Humana Press; New York, NY, USA: 2019.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

