Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics
- PMID: 38540153
- PMCID: PMC10968371
- DOI: 10.3390/biomedicines12030540
Rift Valley Fever Virus: An Overview of the Current Status of Diagnostics
Abstract
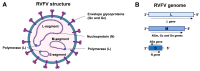
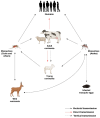
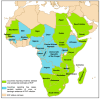
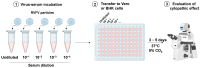
Rift Valley fever is a vector-borne zoonotic disease caused by the Rift Valley fever virus (Phlebovirus genus) listed among the eight pathogens included in the Bluepoint list by the WHO. The transmission is mainly vehicled by Aedes and Culex mosquito species. Symptoms of the disease are varied and non-specific, making clinical diagnosis often challenging, especially in the early stages. Due to the difficulty in distinguishing Rift Valley fever from other viral hemorrhagic fevers, as well as many other diseases that cause fever, an early diagnosis of the infection is important to limit its spread and to provide appropriate care to patients. To date, there is no validated point-of-care diagnostic tool. The virus can only be detected in the blood for a brief period, suggesting that molecular methods alone are not sufficient for case determination. For this, it is preferable to combine both molecular and serological tests. The wide distribution of competent vectors in non-endemic areas, together with global climate change, elicit the spread of RVFV to continents other than Africa, making surveillance activities vital to prevent or to limit the impact of human outbreaks and for a rapid identification of positive cases, making diagnosis a key factor for this achievement.
Keywords: Rift Valley fever virus; early diagnosis; molecular diagnostics; serology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
A geographical information system-based multicriteria evaluation to map areas at risk for Rift Valley fever vector-borne transmission in Italy.Transbound Emerg Dis. 2013 Nov;60 Suppl 2:14-23. doi: 10.1111/tbed.12156. Transbound Emerg Dis. 2013. PMID: 24589097
-
Rift Valley fever virus and European mosquitoes: vector competence of Culex pipiens and Stegomyia albopicta (= Aedes albopictus).Med Vet Entomol. 2017 Dec;31(4):365-372. doi: 10.1111/mve.12254. Epub 2017 Aug 7. Med Vet Entomol. 2017. PMID: 28782121
-
Culex flavivirus infection in a Culex pipiens mosquito colony and its effects on vector competence for Rift Valley fever phlebovirus.Parasit Vectors. 2018 May 23;11(1):310. doi: 10.1186/s13071-018-2887-4. Parasit Vectors. 2018. PMID: 29792223 Free PMC article.
-
Rift Valley Fever: An Emerging Mosquito-Borne Disease.Annu Rev Entomol. 2016;61:395-415. doi: 10.1146/annurev-ento-010715-023819. Annu Rev Entomol. 2016. PMID: 26982443 Review.
-
Current Status of Rift Valley Fever Vaccine Development.Vaccines (Basel). 2017 Sep 19;5(3):29. doi: 10.3390/vaccines5030029. Vaccines (Basel). 2017. PMID: 28925970 Free PMC article. Review.
Cited by
-
Diagnostic serology test comparison for Q fever and Rift Valley fever in humans and livestock from pastoral communities.PLoS Negl Trop Dis. 2024 Oct 14;18(10):e0012300. doi: 10.1371/journal.pntd.0012300. eCollection 2024 Oct. PLoS Negl Trop Dis. 2024. PMID: 39401261 Free PMC article.
References
-
- Daubney R., Hudson J.R., Garnham P.C. Enzootic Hepatitis or Rift Valley Fever. An Undescribed Virus Disease of Sheep Cattle and Man from East Africa. J. Pathol. Bacteriol. 1931;34:545–579. doi: 10.1002/path.1700340418. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

