Lupus susceptibility gene Pbx1 controls the development, stability, and function of regulatory T cells via Rtkn2 expression
- PMID: 38536923
- PMCID: PMC10971436
- DOI: 10.1126/sciadv.adi4310
Lupus susceptibility gene Pbx1 controls the development, stability, and function of regulatory T cells via Rtkn2 expression
Abstract
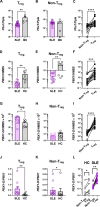
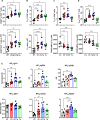
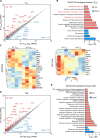
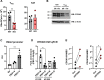
The maintenance of regulatory T (Treg) cells critically prevents autoimmunity. Pre-B cell leukemia transcription factor 1 (Pbx1) variants are associated with lupus susceptibility, particularly through the expression of a dominant negative isoform Pbx1-d in CD4+ T cells. Pbx1-d overexpression impaired Treg cell homeostasis and promoted inflammatory CD4+ T cells. Here, we showed a high expression of Pbx1 in human and murine Treg cells, which is decreased in lupus patients and mice. Pbx1 deficiency or Pbx1-d overexpression reduced the number, stability, and suppressive activity of Treg cells, which increased murine responses to immunization and autoimmune induction. Mechanistically, Pbx1 deficiency altered the expression of genes implicated in cell cycle and apoptosis in Treg cells. Intriguingly, Rtkn2, a Rho-GTPase previously associated with Treg homeostasis, was directly transactivated by Pbx1. Our results suggest that the maintenance of Treg cell homeostasis and stability by Pbx1 through cell cycle progression prevent the expansion of inflammatory T cells that otherwise exacerbates lupus progression in the hosts.
Figures




Similar articles
-
Regulation of the STAT3 pathway by lupus susceptibility gene Pbx1 in T cells.Mol Immunol. 2024 Jan;165:1-10. doi: 10.1016/j.molimm.2023.11.008. Epub 2023 Dec 6. Mol Immunol. 2024. PMID: 38056350
-
The PBX1 lupus susceptibility gene regulates CD44 expression.Mol Immunol. 2017 May;85:148-154. doi: 10.1016/j.molimm.2017.02.016. Epub 2017 Mar 1. Mol Immunol. 2017. PMID: 28257976 Free PMC article.
-
The Lupus Susceptibility Gene Pbx1 Regulates the Balance between Follicular Helper T Cell and Regulatory T Cell Differentiation.J Immunol. 2016 Jul 15;197(2):458-69. doi: 10.4049/jimmunol.1502283. Epub 2016 Jun 13. J Immunol. 2016. PMID: 27296664 Free PMC article.
-
Genetic Variations Controlling Regulatory T Cell Development and Activity in Mouse Models of Lupus-Like Autoimmunity.Front Immunol. 2022 May 26;13:887489. doi: 10.3389/fimmu.2022.887489. eCollection 2022. Front Immunol. 2022. PMID: 35693798 Free PMC article. Review.
-
Regulatory T cells in systemic lupus erythematosus.Eur J Immunol. 2015 Feb;45(2):344-55. doi: 10.1002/eji.201344280. Epub 2014 Dec 15. Eur J Immunol. 2015. PMID: 25378177 Review.
Cited by
-
Comprehensive summary: the role of PBX1 in development and cancers.Front Cell Dev Biol. 2024 Jul 26;12:1442052. doi: 10.3389/fcell.2024.1442052. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39129784 Free PMC article. Review.
References
-
- Wing K., Sakaguchi S., Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nat. Immunol. 11, 7–13 (2010). - PubMed
-
- Jordan M. S., Boesteanu A., Reed A. J., Petrone A. L., Holenbeck A. E., Lerman M. A., Naji A., Caton A. J., Thymic selection of CD4+CD25+ regulatory T cells induced by an agonist self-peptide. Nat. Immunol. 2, 301–306 (2001). - PubMed
-
- Ohkura N., Hamaguchi M., Morikawa H., Sugimura K., Tanaka A., Ito Y., Osaki M., Tanaka Y., Yamashita R., Nakano N., Huehn J., Fehling H. J., Sparwasser T., Nakai K., Sakaguchi S., T cell receptor stimulation-induced epigenetic changes and Foxp3 expression are independent and complementary events required for Treg cell development. Immunity 37, 785–799 (2012). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

