Maintenance of persistent transmission of a plant arbovirus in its insect vector mediated by the Toll-Dorsal immune pathway
- PMID: 38536757
- PMCID: PMC10998634
- DOI: 10.1073/pnas.2315982121
Maintenance of persistent transmission of a plant arbovirus in its insect vector mediated by the Toll-Dorsal immune pathway
Abstract
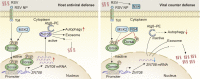
Throughout evolution, arboviruses have developed various strategies to counteract the host's innate immune defenses to maintain persistent transmission. Recent studies have shown that, in addition to bacteria and fungi, the innate Toll-Dorsal immune system also plays an essential role in preventing viral infections in invertebrates. However, whether the classical Toll immune pathway is involved in maintaining the homeostatic process to ensure the persistent and propagative transmission of arboviruses in insect vectors remain unclear. In this study, we revealed that the transcription factor Dorsal is actively involved in the antiviral defense of an insect vector (Laodelphax striatellus) by regulating the target gene, zinc finger protein 708 (LsZN708), which mediates downstream immune-related effectors against infection with the plant virus (Rice stripe virus, RSV). In contrast, an antidefense strategy involving the use of the nonstructural-protein (NS4) to antagonize host antiviral defense through competitive binding to Dorsal from the MSK2 kinase was employed by RSV; this competitive binding inhibited Dorsal phosphorylation and reduced the antiviral response of the host insect. Our study revealed the molecular mechanism through which Toll-Dorsal-ZN708 mediates the maintenance of an arbovirus homeostasis in insect vectors. Specifically, ZN708 is a newly documented zinc finger protein targeted by Dorsal that mediates the downstream antiviral response. This study will contribute to our understanding of the successful transmission and spread of arboviruses in plant or invertebrate hosts.
Keywords: Laodelphax striatellus; Rice stripe virus; dorsal; toll immune pathway; zinc finger protein.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures






Similar articles
-
Arboviruses antagonize insect Toll antiviral immune signaling to facilitate the coexistence of viruses with their vectors.PLoS Pathog. 2024 Jun 12;20(6):e1012318. doi: 10.1371/journal.ppat.1012318. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38865374 Free PMC article.
-
Artificial feeding Rice stripe virus enables efficient virus infection of Laodelphax striatellus.J Virol Methods. 2016 Sep;235:139-143. doi: 10.1016/j.jviromet.2016.06.003. Epub 2016 Jun 6. J Virol Methods. 2016. PMID: 27283882
-
Different pathogenicities of Rice stripe virus from the insect vector and from viruliferous plants.New Phytol. 2016 Apr;210(1):196-207. doi: 10.1111/nph.13747. Epub 2015 Nov 20. New Phytol. 2016. PMID: 26585422 Free PMC article.
-
The small brown planthopper (Laodelphax striatellus) as a vector of the rice stripe virus.Arch Insect Biochem Physiol. 2023 Feb;112(2):e21992. doi: 10.1002/arch.21992. Epub 2022 Dec 27. Arch Insect Biochem Physiol. 2023. PMID: 36575628 Review.
-
New insights on the transmission mechanism of tenuiviruses by their vector insects.Curr Opin Virol. 2018 Dec;33:13-17. doi: 10.1016/j.coviro.2018.07.004. Epub 2018 Jul 18. Curr Opin Virol. 2018. PMID: 30029017 Review.
Cited by
-
Arboviruses antagonize insect Toll antiviral immune signaling to facilitate the coexistence of viruses with their vectors.PLoS Pathog. 2024 Jun 12;20(6):e1012318. doi: 10.1371/journal.ppat.1012318. eCollection 2024 Jun. PLoS Pathog. 2024. PMID: 38865374 Free PMC article.
References
-
- Janeway C. A. Jr., Medzhitov R., Innate immune recognition. Annu. Rev. Immunol. 20, 197–216 (2002). - PubMed
-
- Bang I. S., JAK/STAT signaling in insect innate immunity. Entomol. Res. 49, 339–353 (2019).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

