Contractile and Genetic Characterization of Cardiac Constructs Engineered from Human Induced Pluripotent Stem Cells: Modeling of Tuberous Sclerosis Complex and the Effects of Rapamycin
- PMID: 38534508
- PMCID: PMC10968530
- DOI: 10.3390/bioengineering11030234
Contractile and Genetic Characterization of Cardiac Constructs Engineered from Human Induced Pluripotent Stem Cells: Modeling of Tuberous Sclerosis Complex and the Effects of Rapamycin
Abstract
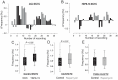
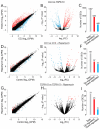
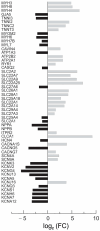
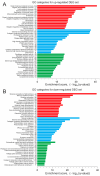
The implementation of three-dimensional tissue engineering concurrently with stem cell technology holds great promise for in vitro research in pharmacology and toxicology and modeling cardiac diseases, particularly for rare genetic and pediatric diseases for which animal models, immortal cell lines, and biopsy samples are unavailable. It also allows for a rapid assessment of phenotype-genotype relationships and tissue response to pharmacological manipulation. Mutations in the TSC1 and TSC2 genes lead to dysfunctional mTOR signaling and cause tuberous sclerosis complex (TSC), a genetic disorder that affects multiple organ systems, principally the brain, heart, skin, and kidneys. Here we differentiated healthy (CC3) and tuberous sclerosis (TSP8-15) human induced pluripotent stem cells (hiPSCs) into cardiomyocytes to create engineered cardiac tissue constructs (ECTCs). We investigated and compared their mechano-elastic properties and gene expression and assessed the effects of rapamycin, a potent inhibitor of the mechanistic target of rapamycin (mTOR). The TSP8-15 ECTCs had increased chronotropy compared to healthy ECTCs. Rapamycin induced positive inotropic and chronotropic effects (i.e., increased contractility and beating frequency, respectively) in the CC3 ECTCs but did not cause significant changes in the TSP8-15 ECTCs. A differential gene expression analysis revealed 926 up- and 439 down-regulated genes in the TSP8-15 ECTCs compared to their healthy counterparts. The application of rapamycin initiated the differential expression of 101 and 31 genes in the CC3 and TSP8-15 ECTCs, respectively. A gene ontology analysis showed that in the CC3 ECTCs, the positive inotropic and chronotropic effects of rapamycin correlated with positively regulated biological processes, which were primarily related to the metabolism of lipids and fatty and amino acids, and with negatively regulated processes, which were predominantly associated with cell proliferation and muscle and tissue development. In conclusion, this study describes for the first time an in vitro TSC cardiac tissue model, illustrates the response of normal and TSC ECTCs to rapamycin, and provides new insights into the mechanisms of TSC.
Keywords: I-Wire cardiac construct; heart-on-a-chip; hiPSC; mTOR; rapamycin; tuberous sclerosis complex.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
TSC patient-derived isogenic neural progenitor cells reveal altered early neurodevelopmental phenotypes and rapamycin-induced MNK-eIF4E signaling.Mol Autism. 2020 Jan 6;11(1):2. doi: 10.1186/s13229-019-0311-3. eCollection 2020. Mol Autism. 2020. PMID: 31921404 Free PMC article.
-
I-Wire Heart-on-a-Chip I: Three-dimensional cardiac tissue constructs for physiology and pharmacology.Acta Biomater. 2017 Jan 15;48:68-78. doi: 10.1016/j.actbio.2016.11.009. Epub 2016 Nov 4. Acta Biomater. 2017. PMID: 27818308 Free PMC article.
-
Tuberous Sclerosis Complex (TSC) Inactivation Increases Neuronal Network Activity by Enhancing Ca2+ Influx via L-Type Ca2+ Channels.J Neurosci. 2021 Sep 29;41(39):8134-8149. doi: 10.1523/JNEUROSCI.1930-20.2021. Epub 2021 Aug 20. J Neurosci. 2021. PMID: 34417327 Free PMC article.
-
Mourning Dr. Alfred G. Knudson: the two-hit hypothesis, tumor suppressor genes, and the tuberous sclerosis complex.Cancer Sci. 2017 Jan;108(1):5-11. doi: 10.1111/cas.13116. Epub 2017 Jan 23. Cancer Sci. 2017. PMID: 27862655 Free PMC article. Review.
-
Mechanistic target of rapamycin (mTOR) in tuberous sclerosis complex-associated epilepsy.Pediatr Neurol. 2015 Mar;52(3):281-9. doi: 10.1016/j.pediatrneurol.2014.10.028. Epub 2014 Nov 20. Pediatr Neurol. 2015. PMID: 25591831 Review.
References
-
- Tohyama S., Fujita J., Fujita C., Yamaguchi M., Kanaami S., Ohno R., Sakamoto K., Kodama M., Kurokawa J., Kanazawa H., et al. Efficient Large-Scale 2D Culture System for Human Induced Pluripotent Stem Cells and Differentiated Cardiomyocytes. Stem Cell Rep. 2017;9:1406–1414. doi: 10.1016/j.stemcr.2017.08.025. - DOI - PMC - PubMed
Grants and funding
- A68485/NH/NIH HHS/United States
- RR030956/NH/NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- DK58404/NH/NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- DK20593/NH/NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- 1S10OD021630 1/NH/NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- S10 OD021630/OD/NIH HHS/United States
- RR024975/NH/NIH HHS/United States
- DK59637/NH/NIH HHS/United States
- G20 RR030956/RR/NCRR NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- UH3TR002097,/NH/NIH HHS/United States
- UH3 TR002097/TR/NCATS NIH HHS/United States
- UL1 RR024975/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

