Wnt5a/β-catenin-mediated epithelial-mesenchymal transition: a key driver of subretinal fibrosis in neovascular age-related macular degeneration
- PMID: 38532410
- PMCID: PMC10967154
- DOI: 10.1186/s12974-024-03068-w
Wnt5a/β-catenin-mediated epithelial-mesenchymal transition: a key driver of subretinal fibrosis in neovascular age-related macular degeneration
Abstract
Background: Neovascular age-related macular degeneration (nAMD), accounts for up to 90% of AMD-associated vision loss, ultimately resulting in the formation of fibrotic scar in the macular region. The pathogenesis of subretinal fibrosis in nAMD involves the process of epithelial-mesenchymal transition (EMT) occurring in retinal pigment epithelium (RPE). Here, we aim to investigate the underlying mechanisms involved in the Wnt signaling during the EMT of RPE cells and in the pathological process of subretinal fibrosis secondary to nAMD.
Methods: In vivo, the induction of subretinal fibrosis was performed in male C57BL/6J mice through laser photocoagulation. Either FH535 (a β-catenin inhibitor) or Box5 (a Wnt5a inhibitor) was intravitreally administered on the same day or 14 days following laser induction. The RPE-Bruch's membrane-choriocapillaris complex (RBCC) tissues were collected and subjected to Western blot analysis and immunofluorescence to examine fibrovascular and Wnt-related markers. In vitro, transforming growth factor beta 1 (TGFβ1)-treated ARPE-19 cells were co-incubated with or without FH535, Foxy-5 (a Wnt5a-mimicking peptide), Box5, or Wnt5a shRNA, respectively. The changes in EMT- and Wnt-related signaling molecules, as well as cell functions were assessed using qRT-PCR, nuclear-cytoplasmic fractionation assay, Western blot, immunofluorescence, scratch assay or transwell migration assay. The cell viability of ARPE-19 cells was determined using Cell Counting Kit (CCK)-8.
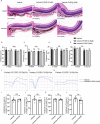
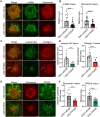
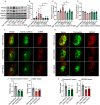
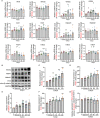
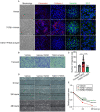
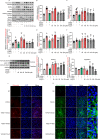
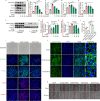
Results: The in vivo analysis demonstrated Wnt5a/ROR1, but not Wnt3a, was upregulated in the RBCCs of the laser-induced CNV mice compared to the normal control group. Intravitreal injection of FH535 effectively reduced Wnt5a protein expression. Both FH535 and Box5 effectively attenuated subretinal fibrosis and EMT, as well as the activation of β-catenin in laser-induced CNV mice, as evidenced by the significant reduction in areas positive for fibronectin, alpha-smooth muscle actin (α-SMA), collagen I, and active β-catenin labeling. In vitro, Wnt5a/ROR1, active β-catenin, and some other Wnt signaling molecules were upregulated in the TGFβ1-induced EMT cell model using ARPE-19 cells. Co-treatment with FH535, Box5, or Wnt5a shRNA markedly suppressed the activation of Wnt5a, nuclear translocation of active β-catenin, as well as the EMT in TGFβ1-treated ARPE-19 cells. Conversely, treatment with Foxy-5 independently resulted in the activation of abovementioned molecules and subsequent induction of EMT in ARPE-19 cells.
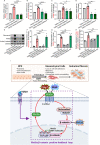
Conclusions: Our study reveals a reciprocal activation between Wnt5a and β-catenin to mediate EMT as a pivotal driver of subretinal fibrosis in nAMD. This positive feedback loop provides valuable insights into potential therapeutic strategies to treat subretinal fibrosis in nAMD patients.
Keywords: Epithelial–mesenchymal transition; Neovascular age-related macular degeneration; Retinal pigment epithelium; Subretinal fibrosis; Wnt5a/β-catenin.
© 2024. The Author(s).
Conflict of interest statement
The authors have no conflicting financial interests.
Figures
Similar articles
-
Luteolin inhibits subretinal fibrosis and epithelial-mesenchymal transition in laser-induced mouse model via suppression of Smad2/3 and YAP signaling.Phytomedicine. 2023 Jul 25;116:154865. doi: 10.1016/j.phymed.2023.154865. Epub 2023 May 9. Phytomedicine. 2023. PMID: 37201365
-
Complement activation contributes to subretinal fibrosis through the induction of epithelial-to-mesenchymal transition (EMT) in retinal pigment epithelial cells.J Neuroinflammation. 2022 Jul 14;19(1):182. doi: 10.1186/s12974-022-02546-3. J Neuroinflammation. 2022. PMID: 35831910 Free PMC article.
-
Macrophage to myofibroblast transition contributes to subretinal fibrosis secondary to neovascular age-related macular degeneration.J Neuroinflammation. 2020 Nov 25;17(1):355. doi: 10.1186/s12974-020-02033-7. J Neuroinflammation. 2020. PMID: 33239022 Free PMC article.
-
Molecular pathogenesis of subretinal fibrosis in neovascular AMD focusing on epithelial-mesenchymal transformation of retinal pigment epithelium.Neurobiol Dis. 2023 Sep;185:106250. doi: 10.1016/j.nbd.2023.106250. Epub 2023 Aug 2. Neurobiol Dis. 2023. PMID: 37536385 Review.
-
EMT and EndMT: Emerging Roles in Age-Related Macular Degeneration.Int J Mol Sci. 2020 Jun 16;21(12):4271. doi: 10.3390/ijms21124271. Int J Mol Sci. 2020. PMID: 32560057 Free PMC article. Review.
Cited by
-
Wnt5a-mediated autophagy contributes to the epithelial-mesenchymal transition of human bronchial epithelial cells during asthma.Mol Med. 2024 Jun 19;30(1):93. doi: 10.1186/s10020-024-00862-3. Mol Med. 2024. PMID: 38898476 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

