IFN-λ drives distinct lung immune landscape changes and antiviral responses in human metapneumovirus infection
- PMID: 38530032
- PMCID: PMC11077986
- DOI: 10.1128/mbio.00550-24
IFN-λ drives distinct lung immune landscape changes and antiviral responses in human metapneumovirus infection
Abstract
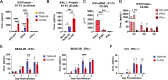
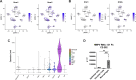
Human metapneumovirus (HMPV) is a primary cause of acute respiratory infection, yet there are no approved vaccines or antiviral therapies for HMPV. Early host responses to HMPV are poorly characterized, and further understanding could identify important antiviral pathways. Type III interferon (IFN-λ) displays potent antiviral activity against respiratory viruses and is being investigated for therapeutic use. However, its role in HMPV infection remains largely unknown. Here, we show that IFN-λ is highly upregulated during HMPV infection in vitro in human and mouse airway epithelial cells and in vivo in mice. We found through several immunological and molecular assays that type II alveolar cells are the primary producers of IFN-λ. Using mouse models, we show that IFN-λ limits lung HMPV replication and restricts virus spread from upper to lower airways but does not contribute to clinical disease. Moreover, we show that IFN-λ signaling is predominantly mediated by CD45- non-immune cells. Mice lacking IFN-λ signaling showed diminished loss of ciliated epithelial cells and decreased recruitment of lung macrophages in early HMPV infection along with higher inflammatory cytokine and interferon-stimulated gene expression, suggesting that IFN-λ may maintain immunomodulatory responses. Administration of IFN-λ for prophylaxis or post-infection treatment in mice reduced viral load without inflammation-driven weight loss or clinical disease. These data offer clinical promise for IFN-λ in HMPV treatment.
Importance: Human metapneumovirus (HMPV) is a common respiratory pathogen and often contributes to severe disease, particularly in children, immunocompromised people, and the elderly. There are currently no licensed HMPV antiviral treatments or vaccines. Here, we report novel roles of host factor IFN-λ in HMPV disease that highlight therapeutic potential. We show that IFN-λ promotes lung antiviral responses by restricting lung HMPV replication and spread from upper to lower airways but does so without inducing lung immunopathology. Our data uncover recruitment of lung macrophages, regulation of ciliated epithelial cells, and modulation of inflammatory cytokines and interferon-stimulated genes as likely contributors. Moreover, we found these roles to be distinct and non-redundant, as they are not observed with knockout of, or treatment with, type I IFN. These data elucidate unique antiviral functions of IFN-λ and suggest IFN-λ augmentation as a promising therapeutic for treating HMPV disease and promoting effective vaccine responses.
Keywords: host-pathogen immunity; human metapneumovirus; interferon; respiratory infection.
Conflict of interest statement
J.V.W. previously served on the Scientific Advisory Board of Quidel and an Independent Data Monitoring Committee for GlaxoSmithKline, neither activity involved with the work under consideration. All other authors declare no conflicts of interest.
Figures






Similar articles
-
Impact and regulation of lambda interferon response in human metapneumovirus infection.J Virol. 2015 Jan;89(1):730-42. doi: 10.1128/JVI.02897-14. Epub 2014 Oct 29. J Virol. 2015. PMID: 25355870 Free PMC article.
-
Alveolar macrophages contribute to the pathogenesis of human metapneumovirus infection while protecting against respiratory syncytial virus infection.Am J Respir Cell Mol Biol. 2014 Oct;51(4):502-15. doi: 10.1165/rcmb.2013-0414OC. Am J Respir Cell Mol Biol. 2014. PMID: 24749674 Free PMC article.
-
Role of type I interferon signaling in human metapneumovirus pathogenesis and control of viral replication.J Virol. 2015 Apr;89(8):4405-20. doi: 10.1128/JVI.03275-14. Epub 2015 Feb 4. J Virol. 2015. PMID: 25653440 Free PMC article.
-
Interferon-Mediated Response to Human Metapneumovirus Infection.Viruses. 2018 Sep 18;10(9):505. doi: 10.3390/v10090505. Viruses. 2018. PMID: 30231515 Free PMC article. Review.
-
Cell-Mediated Responses to Human Metapneumovirus Infection.Viruses. 2020 May 14;12(5):542. doi: 10.3390/v12050542. Viruses. 2020. PMID: 32423043 Free PMC article. Review.
Cited by
-
IFN-λ uniquely promotes CD8 T cell immunity against SARS-CoV-2 relative to type I IFN.JCI Insight. 2024 May 21;9(13):e171830. doi: 10.1172/jci.insight.171830. JCI Insight. 2024. PMID: 38973611 Free PMC article.
References
-
- Levels and trends in child mortality. 2019. Available from: https://www.unicef.org/reports/levels-and-trends-child-mortality-report-.... Retrieved 1 Apr 2021.
-
- Pneumonia. Available from: https://www.who.int/news-room/fact-sheets/detail/pneumonia. Retrieved 1 Apr 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
