Genetic loci regulate Sarbecovirus pathogenesis: A comparison across mice and humans
- PMID: 38508400
- PMCID: PMC10981091
- DOI: 10.1016/j.virusres.2024.199357
Genetic loci regulate Sarbecovirus pathogenesis: A comparison across mice and humans
Abstract
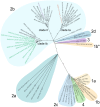
Coronavirus (CoV) cause considerable morbidity and mortality in humans and other mammals, as evidenced by the emergence of Severe Acute Respiratory CoV (SARS-CoV) in 2003, Middle East Respiratory CoV (MERS-CoV) in 2012, and SARS-CoV-2 in 2019. Although poorly characterized, natural genetic variation in human and other mammals modulate virus pathogenesis, as reflected by the spectrum of clinical outcomes ranging from asymptomatic infections to lethal disease. Using multiple human epidemic and zoonotic Sarbecoviruses, coupled with murine Collaborative Cross genetic reference populations, we identify several dozen quantitative trait loci that regulate SARS-like group-2B CoV pathogenesis and replication. Under a Chr4 QTL, we deleted a candidate interferon stimulated gene, Trim14 which resulted in enhanced SARS-CoV titers and clinical disease, suggesting an antiviral role during infection. Importantly, about 60 % of the murine QTL encode susceptibility genes identified as priority candidates from human genome-wide association studies (GWAS) studies after SARS-CoV-2 infection, suggesting that similar selective forces have targeted analogous genes and pathways to regulate Sarbecovirus disease across diverse mammalian hosts. These studies provide an experimental platform in rodents to investigate the molecular-genetic mechanisms by which potential cross mammalian susceptibility loci and genes regulate type-specific and cross-SARS-like group 2B CoV replication, immunity, and pathogenesis in rodent models. Our study also provides a paradigm for identifying susceptibility loci for other highly heterogeneous and virulent viruses that sporadically emerge from zoonotic reservoirs to plague human and animal populations.
Keywords: Host susceptibility loci; Pathogenesis; SARS-CoV; SARS-CoV-2; Zoonotic CoV.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
A Multitrait Locus Regulates Sarbecovirus Pathogenesis.mBio. 2022 Aug 30;13(4):e0145422. doi: 10.1128/mbio.01454-22. Epub 2022 Jul 12. mBio. 2022. PMID: 35862771 Free PMC article.
-
Sarbecovirus disease susceptibility is conserved across viral and host models.Virus Res. 2024 Aug;346:199399. doi: 10.1016/j.virusres.2024.199399. Epub 2024 Jun 14. Virus Res. 2024. PMID: 38823688 Free PMC article.
-
Common Mechanism of SARS-CoV and SARS-CoV-2 Pathogenesis across Species.bioRxiv [Preprint]. 2021 May 14:2021.05.14.444205. doi: 10.1101/2021.05.14.444205. bioRxiv. 2021. PMID: 34013261 Free PMC article. Preprint.
-
Broad-spectrum coronavirus antiviral drug discovery.Expert Opin Drug Discov. 2019 Apr;14(4):397-412. doi: 10.1080/17460441.2019.1581171. Epub 2019 Mar 8. Expert Opin Drug Discov. 2019. PMID: 30849247 Free PMC article. Review.
-
SARS-CoV-2, the pandemic coronavirus: Molecular and structural insights.J Basic Microbiol. 2021 Mar;61(3):180-202. doi: 10.1002/jobm.202000537. Epub 2021 Jan 18. J Basic Microbiol. 2021. PMID: 33460172 Free PMC article. Review.
Cited by
-
Mapping of susceptibility loci for Ebola virus pathogenesis in mice.Cell Rep. 2024 May 28;43(5):114127. doi: 10.1016/j.celrep.2024.114127. Epub 2024 Apr 21. Cell Rep. 2024. PMID: 38652660 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

