CK2 activity is crucial for proper glucagon expression
- PMID: 38503901
- PMCID: PMC11153270
- DOI: 10.1007/s00125-024-06128-1
CK2 activity is crucial for proper glucagon expression
Abstract
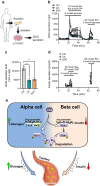
Aims/hypothesis: Protein kinase CK2 acts as a negative regulator of insulin expression in pancreatic beta cells. This action is mainly mediated by phosphorylation of the transcription factor pancreatic and duodenal homeobox protein 1 (PDX1). In pancreatic alpha cells, PDX1 acts in a reciprocal fashion on glucagon (GCG) expression. Therefore, we hypothesised that CK2 might positively regulate GCG expression in pancreatic alpha cells.
Methods: We suppressed CK2 kinase activity in αTC1 cells by two pharmacological inhibitors and by the CRISPR/Cas9 technique. Subsequently, we analysed GCG expression and secretion by real-time quantitative RT-PCR, western blot, luciferase assay, ELISA and DNA pull-down assays. We additionally studied paracrine effects on GCG secretion in pseudoislets, isolated murine islets and human islets. In vivo, we examined the effect of CK2 inhibition on blood glucose levels by systemic and alpha cell-specific CK2 inhibition.
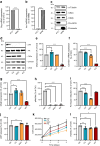
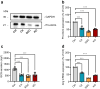
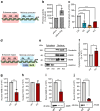
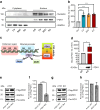
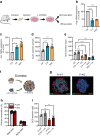
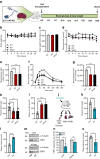
Results: We found that CK2 downregulation reduces GCG secretion in the murine alpha cell line αTC1 (e.g. from 1094±124 ng/l to 459±110 ng/l) by the use of the CK2-inhibitor SGC-CK2-1. This was due to a marked decrease in Gcg gene expression through alteration of the binding of paired box protein 6 (PAX6) and transcription factor MafB to the Gcg promoter. The analysis of the underlying mechanisms revealed that both transcription factors are displaced by PDX1. Ex vivo experiments in isolated murine islets and pseudoislets further demonstrated that CK2-mediated reduction in GCG secretion was only slightly affected by the higher insulin secretion after CK2 inhibition. The kidney capsule transplantation model showed the significance of CK2 for GCG expression and secretion in vivo. Finally, CK2 downregulation also reduced the GCG secretion in islets isolated from humans.
Conclusions/interpretation: These novel findings not only indicate an important function of protein kinase CK2 for proper GCG expression but also demonstrate that CK2 may be a promising target for the development of novel glucose-lowering drugs.
Keywords: Glucagon; Glucose homeostasis; PDX1; Pancreatic alpha cells; Protein kinase CK2.
© 2024. The Author(s).
Figures
Similar articles
-
Glucose-dependent downregulation of glucagon gene expression mediated by selective interactions between ALX3 and PAX6 in mouse alpha cells.Diabetologia. 2016 Apr;59(4):766-75. doi: 10.1007/s00125-015-3849-4. Epub 2016 Jan 6. Diabetologia. 2016. PMID: 26739814
-
CK2 phosphorylation of Pdx-1 regulates its transcription factor activity.Cell Mol Life Sci. 2010 Jul;67(14):2481-9. doi: 10.1007/s00018-010-0348-0. Epub 2010 Mar 26. Cell Mol Life Sci. 2010. PMID: 20339896 Free PMC article.
-
Glucose regulates protein kinase CK2 in pancreatic β-cells and its interaction with PDX-1.Int J Biochem Cell Biol. 2013 Dec;45(12):2786-95. doi: 10.1016/j.biocel.2013.10.002. Epub 2013 Oct 12. Int J Biochem Cell Biol. 2013. PMID: 24126110
-
Mechanisms underlying proglucagon gene expression.J Endocrinol. 2008 Jul;198(1):17-28. doi: 10.1677/JOE-08-0085. J Endocrinol. 2008. PMID: 18577568 Review.
-
Glucagon gene expression in the endocrine pancreas: the role of the transcription factor Pax6 in α-cell differentiation, glucagon biosynthesis and secretion.Diabetes Obes Metab. 2011 Oct;13 Suppl 1:31-8. doi: 10.1111/j.1463-1326.2011.01445.x. Diabetes Obes Metab. 2011. PMID: 21824254 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

