Anti-Müllerian hormone, testicular descent and cryptorchidism
- PMID: 38501100
- PMCID: PMC10944898
- DOI: 10.3389/fendo.2024.1361032
Anti-Müllerian hormone, testicular descent and cryptorchidism
Abstract
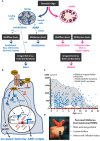
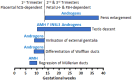
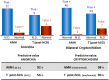
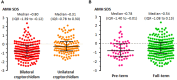
Anti-Müllerian hormone (AMH) is a Sertoli cell-secreted glycoprotein involved in male fetal sex differentiation: it provokes the regression of Müllerian ducts, which otherwise give rise to the Fallopian tubes, the uterus and the upper part of the vagina. In the first trimester of fetal life, AMH is expressed independently of gonadotropins, whereas from the second trimester onwards AMH testicular production is stimulated by FSH and oestrogens; at puberty, AMH expression is inhibited by androgens. AMH has also been suggested to participate in testicular descent during fetal life, but its role remains unclear. Serum AMH is a well-recognized biomarker of testicular function from birth to the first stages of puberty. Especially in boys with nonpalpable gonads, serum AMH is the most useful marker of the existence of testicular tissue. In boys with cryptorchidism, serum AMH levels reflect the mass of functional Sertoli cells: they are lower in patients with bilateral than in those with unilateral cryptorchidism. Interestingly, serum AMH increases after testis relocation to the scrotum, suggesting that the ectopic position result in testicular dysfunction, which may be at least partially reversible. In boys with cryptorchidism associated with micropenis, low AMH and FSH are indicative of central hypogonadism, and serum AMH is a good marker of effective FSH treatment. In patients with cryptorchidism in the context of disorders of sex development, low serum AMH is suggestive of gonadal dysgenesis, whereas normal or high AMH is found in patients with isolated androgen synthesis defects or with androgen insensitivity. In syndromic disorders, assessment of serum AMH has shown that Sertoli cell function is preserved in boys with Klinefelter syndrome until mid-puberty, while it is affected in patients with Noonan, Prader-Willi or Down syndromes.
Keywords: AMH; androgens; anti-Müllerian hormone; cryptorchidism; testicular descent; testis.
Copyright © 2024 Rey and Grinspon.
Conflict of interest statement
Until 2020, RR received royalties for the development of an AMH ELISA kit and both authors received honoraria for technology services using the AMH ELISA. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Anti-Müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis.Int J Pediatr Endocrinol. 2016;2016:20. doi: 10.1186/s13633-016-0038-2. Epub 2016 Oct 28. Int J Pediatr Endocrinol. 2016. PMID: 27799946 Free PMC article. Review.
-
Anti-müllerian hormone and sertoli cell function in paediatric male hypogonadism.Horm Res Paediatr. 2010;73(2):81-92. doi: 10.1159/000277140. Epub 2010 Feb 9. Horm Res Paediatr. 2010. PMID: 20190544 Review.
-
[Anti-Müllerian hormone (AMH) measurements in the assessment of testicular function in prepubertal boys and in sexual differentiation disorders].Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2006;12(3):195-9. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw. 2006. PMID: 17020655 Polish.
-
Anti-müllerian hormone and testosterone serum levels are inversely during normal and precocious pubertal development.J Clin Endocrinol Metab. 1993 Nov;77(5):1220-6. doi: 10.1210/jcem.77.5.8077315. J Clin Endocrinol Metab. 1993. PMID: 8077315
-
Anti-Müllerian Hormone and Testicular Function in Prepubertal Boys With Cryptorchidism.Front Endocrinol (Lausanne). 2018 Apr 25;9:182. doi: 10.3389/fendo.2018.00182. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29922225 Free PMC article.
References
-
- Jost A. Problems of fetal endocrinology: the gonadal and hypophyseal hormones. Recent Prog Hormone Res. (1953) 8:379–418.
-
- Lasala C, Schteingart HF, Arouche N, Bedecarrás P, Grinspon RP, Picard JY, et al. . SOX9 and SF1 are involved in cyclic AMP-mediated upregulation of anti-Mullerian gene expression in the testicular prepubertal Sertoli cell line SMAT1. Am J Physiol Endocrinol Metab. (2011) 301:E539–547. doi: 10.1152/ajpendo.00187.2011 - DOI - PubMed
-
- Grinspon RP, Ropelato MG, Bedecarrás P, Loreti N, Ballerini MG, Gottlieb S, et al. . Gonadotrophin secretion pattern in anorchid boys from birth to pubertal age: pathophysiological aspects and diagnostic usefulness. Clin Endocrinol (Oxf). (2012) 76:698–705. doi: 10.1111/j.1365-2265.2011.04297.x - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

