Intradiscal administration of autologous platelet-rich plasma in patients with Modic type 1 associated low back pain: A prospective pilot study
- PMID: 38500785
- PMCID: PMC10945308
- DOI: 10.1002/jsp2.1320
Intradiscal administration of autologous platelet-rich plasma in patients with Modic type 1 associated low back pain: A prospective pilot study
Abstract
Background: Various treatments for chronic low back pain (LBP) have been reported; among them, platelet-rich plasma (PRP) as a regenerative medicine has attracted much attention. Although Modic type 1 change (MC1) is associated with LBP, no treatment has been established so far. In addition, no studies have administered PRP to intervertebral discs (IVDs) in patients with LBP, targeting MC1 only. Thus, the purpose of this study was to determine the safety and efficacy of PRP administration to the IVDs in patients with MC1 experiencing LBP.
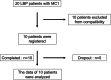
Methods: PRP was injected intradiscally to 10 patients with MC1 experiencing LBP. Patients were followed prospectively for up to 24 weeks after primary administration. Physical condition, laboratory data, and lumbar x-ray images were evaluated for safety assessment. Furthermore, to evaluate the effectiveness of PRP, patient-reported outcomes were considered. In addition, changes in MC1 were assessed using magnetic resonance imaging (MRI).
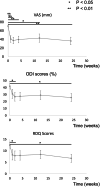
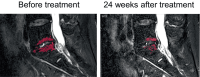
Results: There were no adverse events in the laboratory data or lumbar X-ray images after administration. The mean visual analog scale, which was 70.0 ± 13.3 before the treatment, significantly decreased 1 week after PRP administration and was 39.0 ± 28.8 at the last observation. Oswestry disability index and Roland Morris disability questionnaire scores promptly improved after treatment, and both improved significantly 24 weeks after PRP administration. Follow-up MRI 24 weeks after treatment showed a significant decrease in the mean high-signal intensity of fat-suppressed T2-weighted imaging from 10.1 to 7.90 mm2 compared with that before PRP administration.
Conclusions: The safety and efficacy of PRP administration to the IVDs of patients with MC1 experiencing LBP were identified. Post-treatment MRI suggested improvement in inflammation, speculating that PRP suppressed inflammation and consequently relieved the patient's symptoms. Despite the small number of patients, this treatment is promising for patients with MC1 experiencing LBP. The study protocol has been reviewed and approved by the Certified Committee for Regenerative Medicine and the Japanese Ministry of Health, Labor and Welfare (Japan Registry of Clinical Trials [jRCT] No. jRCTb042210159).
Keywords: Modic type 1change (MC1); intervertebral disc (IVDs); low back pain (LBP); platelet‐rich plasma (PRP); regenerative medicines.
© 2024 The Authors. JOR Spine published by Wiley Periodicals LLC on behalf of Orthopaedic Research Society.
Conflict of interest statement
The research institution received research support from Zimmer Biomet. This study was technically supported by Canon Medical Systems Corporation. Dr. Yoshiharu Ohno has a research grant from Canon Medical Systems Corporation.
Figures
Similar articles
-
Autologous Platelet-Rich Plasma Administration on the Intervertebral Disc in Low Back Pain Patients with Modic Type 1 Change: Report of Two Cases.Medicina (Kaunas). 2023 Jan 5;59(1):112. doi: 10.3390/medicina59010112. Medicina (Kaunas). 2023. PMID: 36676735 Free PMC article.
-
Retrospective Analysis of Factors Associated with the Treatment Outcomes of Intradiscal Platelet-Rich Plasma-Releasate Injection Therapy for Patients with Discogenic Low Back Pain.Medicina (Kaunas). 2023 Mar 23;59(4):640. doi: 10.3390/medicina59040640. Medicina (Kaunas). 2023. PMID: 37109598 Free PMC article. Clinical Trial.
-
Intradiscal Injection of Autologous Platelet-Rich Plasma Releasate to Treat Discogenic Low Back Pain: A Preliminary Clinical Trial.Asian Spine J. 2017 Jun;11(3):380-389. doi: 10.4184/asj.2017.11.3.380. Epub 2017 Jun 15. Asian Spine J. 2017. PMID: 28670405 Free PMC article.
-
Platelet-rich plasma in the management of chronic low back pain: a critical review.J Pain Res. 2019 Feb 25;12:753-767. doi: 10.2147/JPR.S153085. eCollection 2019. J Pain Res. 2019. PMID: 30881089 Free PMC article. Review.
-
Platelet-Rich Plasma Treatment for the Lumbar Spine: A Review and Discussion of Existing Gaps.Pain Physician. 2024 Jul;27(5):283-302. Pain Physician. 2024. PMID: 39087964 Review.
Cited by
-
Discogenic Low Back Pain: Anatomic and Pathophysiologic Characterization, Clinical Evaluation, Biomarkers, AI, and Treatment Options.J Clin Med. 2024 Oct 3;13(19):5915. doi: 10.3390/jcm13195915. J Clin Med. 2024. PMID: 39407975 Free PMC article. Review.
References
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators , James SL, Abate D, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990‐2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1545‐1602. doi:10.1016/s0140-6736(16)31678-6 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

